This research paper explores the synthesis, characterization, and applications of zinc oxide (\(TiO_2-ZnO\)) nanomaterials, with a focus on their antimicrobial properties and potential use in textiles. The study details the successful synthesis of \(TiO_2-ZnO\) nanoparticles using the traditional Ayurvedic method of Bhasmikaran, a process involving purification and incineration to transform zinc into a bioavailable form. The resulting nanomaterials were characterized using a range of modern analytical techniques, including DRS-UV-visible spectroscopy, X-ray diffraction (XRD), scanning electron microscopy (SEM), energy dispersive X-ray spectroscopy (EDX), Fourier transform infrared spectroscopy (FT-IR), Raman spectroscopy, photoluminescence spectroscopy, and transmission electron microscopy (TEM). The antimicrobial activity of the synthesized \(TiO_2-ZnO\) nanoparticles was evaluated, demonstrating their effectiveness against various microorganisms, which is crucial for textile applications. Furthermore, the study investigated the application of \(TiO_2-ZnO\) nanomaterials in textiles by assessing their impact on crease recovery, stiffness, and tearing strength, highlighting the potential of \(TiO_2-ZnO\) to enhance fabric performance and impart antimicrobial properties.
The synergetic effectiveness of nanomaterials, particularly titanium dioxide-zinc oxide (TiO\({}_{2}\)-ZnO) nanocomposites, in antimicrobial activity presents a promising avenue for advanced textile applications. The combination of TiO\(_2\) and ZnO leverages the unique properties of each component, resulting in enhanced antimicrobial performance compared to individual nanomaterials. TiO\({}_{2}\), upon UV irradiation, generates reactive oxygen species (ROS) that damage bacterial cell membranes, while ZnO exhibits antimicrobial activity through the release of zinc ions and ROS generation, even in the absence of UV light. The synergetic effect arises from the complementary mechanisms of these nanomaterials. TiO\({}_{2}\) enhances the photocatalytic activity, facilitating efficient ROS generation, while ZnO’s broader activity spectrum ensures antimicrobial efficacy under various conditions. This combination offers a dual-action approach, addressing a wider range of microorganisms and mitigating potential resistance development [1– 9].
In textile applications, TiO\({}_{2}\)-ZnO nanocomposites can be incorporated via padding, coating, or fiber modification, imparting durable antimicrobial properties. These treated textiles find applications in healthcare, sportswear, and protective clothing, where microbial control is crucial. The nanocomposites’ ability to inhibit bacterial growth and odor generation enhances the hygiene and longevity of textiles, contributing to improved user comfort and safety.
Furthermore, the synergetic effect of TiO\({}_{2}\)-ZnO nanocomposites extends to self-cleaning and UV-protective properties, adding multifunctional benefits to textiles. The photocatalytic activity of TiO\({}_{2}\) aids in the degradation of organic pollutants, while both materials offer UV absorption, safeguarding the fabric and the user from harmful UV radiation. The development of TiO\({}_{2}\)-ZnO nanocomposite-treated textiles offers a sustainable and efficient approach to antimicrobial applications, addressing the growing demand for hygienic and functional textiles. The synergetic effectiveness of these nanomaterials provides a versatile platform for innovation in textile technology [10– 17].
Sudhir Arbuj et al. propose a synthesis of TiO\({}_{2}\) nanoparticles by the simple precipitation method using titanium chloride as a precursor. Initially, one mole of titanium chloride was added to 35 mL ice-cold DI water in a 500 mL round-bottom flask. 10 mL ammonia solution was added dropwise to the above solution with constant stirring. The resultant product was thoroughly washed with ample distilled water to remove chloride ions. Finally, titanium hydroxide was dried at 110\(\mathrm{{}^\circ}\)C for 2 hours. The resultant powder was converted to TiO\({}_{2}\) nanoparticles by calcination in a muffle furnace at 400\(\mathrm{{}^\circ}\)C for 4 hours [18– 21].
Jasad Bhasma, a zinc-based Ayurvedic formulation, is synthesized through the Bhasmikaran process, which transforms zinc into bioavailable ZnO nanoparticles. The process begins with Shodhan (purification), where raw zinc is repeatedly heated and quenched 21 times in liquids such as cow’s milk, buttermilk, or Triphala decoction to eliminate impurities and enhance therapeutic properties. Following purification, the Marana (incineration) process is carried out. The purified zinc is triturated with herbal extracts like Aloe vera juice to form a fine paste. This paste is then dried and subjected to repeated controlled heating in earthen crucibles (Sharava Samputa) using a traditional furnace (Puta system). Through multiple heating and cooling cycles, metallic zinc undergoes oxidation, leading to the formation of ZnO nanoparticles. Modern analytical techniques such as X-ray diffraction (XRD) and electron microscopy (SEM, TEM) confirm the nanoscale structure and purity of the final product. This Ayurvedic synthesis method ensures high bioavailability and stability, making Jasad Bhasma a potent natural nanomedicine with antimicrobial, wound-healing, and immune-boosting properties [22– 26].
The process commenced with the synthesis of TiO\({}_{2}\) NPs using simple hydrolysis of titanium chloride. The ZnO powder Jasad Bhasma was homogeneously mixed with TiO\({}_{2}\) at concentrations 10% using a mortar and pestle to ensure an even distribution of TiO\(_2\) ions throughout ZnO. The mixture was then thoroughly ground for 3 h to achieve uniformity. Subsequent to this, the composite powder was annealed at 450\(\mathrm{{}^\circ}\)C for 4 h, promote the incorporation of TiO\({}_{2}\) into the ZnO. This resulted in the formation of TiO\({}_{2}\)– ZnO nanocomposites. The synthesized nanocomposites were stored for further characterization and for further potential applications [27– 41].
Characterizing nanomaterials is essential to assess their size, shape, surface charge, and morphology, as they are invisible to the naked eye and require advanced techniques. Various spectroscopic and microscopic methods help confirm their structure and origin. The choice of characterization techniques depends on the intended applications of the nanomaterials. In this study, black-colored particles and fine white powder were obtained. X-ray diffraction (XRD) is used to analyze the crystal structures of TiO\(_2\) nanomaterials, while scanning electron microscopy (SEM) examines their surface morphology. Energy dispersive X-ray spectroscopy (EDAX) identifies the elemental composition of composites. Additionally, UV-Vis-NIR spectroscopy investigates the optical absorbance of TiO\(_2\) within the 200–800 nm wavelength range [42– 45].
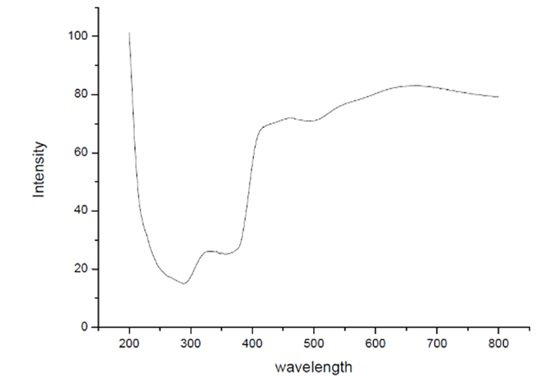
The diffuse reflectance spectroscopy (DRS) UV-Visible analysis of TiO\({}_{2}\)-ZnO nanomaterials was performed at Jaysingpur College, Jaysingpur, India. using a Jasco Spectrophotometer Model V-770. This analysis was conducted to investigate the optical properties of the synthesized TiO\({}_{2}\)-ZnO nanomaterials, specifically their absorption characteristics in the UV-Visible region. Figure 1 depicts DRS-UV-Visible spectroscopic graph of TiO\(_2\)-ZnO\({}_{\ }\)nanocomposite [52– 54].
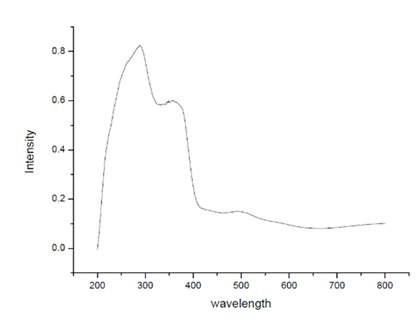
The experiment utilized a D\(_2\)/WI (deuterium/tungsten iodide) light source to ensure accurate spectral measurements across the UV and visible wavelength range. The scan speed was maintained at 400 nm/min to achieve a smooth and precise spectral profile. he TiO\(_2\)-ZnO (Jasad Bhasma) composite exhibits enhanced structural, optical, and functional properties, making it suitable for environmental and biomedical applications. X-ray diffraction (XRD) confirms the crystalline nature of the composite, while scanning electron microscopy (SEM) reveals a uniform dispersion of ZnO within the TiO\(_2\) matrix, increasing surface area and reactive sites. UV-Vis spectroscopy indicates a broad absorption spectrum extending into the visible range, enhancing its photocatalytic efficiency for environmental remediation. The integration of Ayurvedically synthesized Jasad Bhasma-derived ZnO imparts bioactive properties, including antimicrobial effects, wound healing, and biocompatibility, making it useful for biomedical applications. The composite’s high surface area and efficient charge separation further improve its potential in photocatalytic degradation of pollutants, particularly in wastewater treatment. The synergistic combination of TiO\(_2\) and ZnO results in a multifunctional material with superior photocatalytic, antimicrobial, and bioactive characteristics, offering a sustainable and efficient solution for nanomedicine and environmental applications [55– 60].
The X-ray diffraction (XRD) analysis of TiO\(_2\)– ZnO. nanocomposite was carried out using a Bruker D2 Phaser (Germany) diffractometer at room temperature. This study aimed to determine the crystallographic structure, phase composition, and crystallite size of the synthesized ZnO. nanomaterials. A Cu-K\(\alpha\) radiation source (\(\lambda\) = 1.54 Å) was used as the target atom, ensuring high-resolution diffraction patterns. The detection system employed a LYNXEYE detector, which enhances signal accuracy and reduces noise. The scanning was performed with a minimum step size of 0.005\(\mathrm{{}^\circ}\), providing detailed peak resolution for phase identification. The sample was analyzed in PIFC, Shivaji University, Kolhapur, India. The XRD pattern of the synthesized TiO\({}_{2}\)-ZnO. sample is shown below in Figure 2.
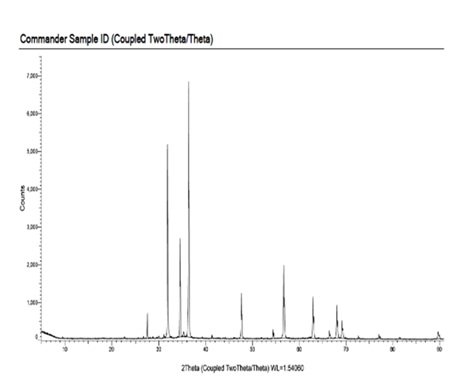
The X-ray diffraction (XRD) pattern of the TiO\(_2\)-ZnO (Jasad Bhasma) nanocomposite reveals distinct crystalline phases, confirming the successful synthesis of the composite material. The diffraction peaks observed at characteristic 2\(\theta\) values correspond to the anatase phase of TiO\(_2\) and the hexagonal wurtzite structure of ZnO. The presence of sharp and intense peaks indicates the high crystallinity of the nanocomposite, which is essential for its enhanced photocatalytic and bioactive properties. The broadening of some peaks suggests the nanoscale nature of the synthesized material, indicating a reduction in crystallite size. The absence of additional impurity peaks confirms the purity of the synthesized TiO\(_2\)-ZnO nanocomposite, validating the effectiveness of the Ayurvedic Bhasmikaran process in obtaining high-purity ZnO from Jasad Bhasma. The synergistic interaction between TiO\(_2\) and ZnO enhances the charge separation efficiency, improving its photocatalytic activity for environmental remediation applications. Furthermore, the structural integrity and phase purity of the composite make it a potential candidate for antimicrobial and wound-healing applications. The XRD analysis confirms the presence of a well-formed TiO\(_2\)-ZnO composite with superior structural and functional properties, making it an effective material for nanomedicine and sustainable applications [61– 64].
The Field Emission Scanning Electron Microscopy (FESEM) analysis of TiO\({}_{2}\)-ZnO nanocomposite was conducted to investigate their surface morphology, particle size, and distribution. The imaging was performed using an ultra-high resolution FESEM, ensuring detailed structural characterization. The sample was analyzed in SPPU, Pune, India. Figure 3 presents the SEM analysis results, highlighting the nanoscale features of the synthesized TiO\(_2\)-ZnO nanomaterials. Figure 3 depicts SEM image of TiO\({}_{2}\)-ZnO nanomaterials.
The microscope operated at multiple accelerating voltages—1.0 nm at 15 kV, 1.4 nm at 1 kV, and 1.8 nm at 3 kV and 30 Pa—allowing for precise imaging across different resolutions. The system was equipped with In-lens TLD (Through-the-Lens Detector), SE (Secondary Electron), and BSE (Backscattered Electron) detectors, which provided comprehensive insights into both the topographical and compositional characteristics of the sample. Additionally, the Load Lock (Quick Loader) system facilitated efficient and contamination-free sample handling, ensuring the accuracy and reliability of the imaging process [65, 70].
The field emission scanning electron microscopy (FESEM) image of the TiO\(_2\)-ZnO (Jasad Bhasma) nanocomposite provides crucial insights into its morphological characteristics. The image, taken at a magnification of 3000\(\mathrm{\times}\), reveals a highly agglomerated structure consisting of irregularly shaped nanoparticles with a rough surface texture. The presence of both spherical and rod-like particles suggests the successful integration of ZnO and TiO\(_2\) phases, with ZnO nanoparticles likely contributing to the smaller granular formations, while TiO\(_2\) exhibits a more aggregated, crystalline structure. The nanoscale dimensions observed in the image indicate a high surface-to-volume ratio, which is beneficial for catalytic and biomedical applications. The uniform dispersion of ZnO within the TiO\(_2\) matrix suggests strong interfacial interaction between the two metal oxides, which can enhance photocatalytic activity and antibacterial properties. The bright contrast in the image corresponds to the denser regions of the composite, while the darker areas may indicate porous structures, facilitating enhanced adsorption capabilities. The observed morphology confirms that the Ayurvedic Bhasmikaran method effectively produces a well-structured nanocomposite with promising functional properties [71– 74].

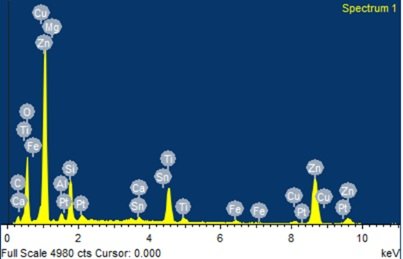
Energy Dispersive X-ray Spectroscopy (EDX) analysis was conducted to determine the elemental composition of the synthesized TiO\(_2\)-ZnO nanocomposite. The measurements were performed using a Bruker XFlash 6I30 EDS detector, which offers excellent energy resolution (123 eV at Mn K\(\alpha\) and 45 eV at C K\(\alpha\)) and an elemental detection range from Be (Z=4) to Am (Z=95). The analysis was controlled using Espirit 1.9 software, ensuring precise elemental quantification. Figure 4 reveals EDX Spectrum of TiO\({}_{2}\)-ZnO nanomaterials.
The energy-dispersive X-ray (EDX) spectrum of the TiO\(_2\)-ZnO (Jasad Bhasma) nanocomposite confirms the elemental composition of the synthesized material. The prominent peaks for Zn and Ti indicate the successful incorporation of zinc oxide (ZnO) and titanium dioxide (TiO\(_2\)) into the nanocomposite. The presence of oxygen (O) confirms the formation of metal oxides, supporting the oxidation of Zn and Ti during synthesis. Additional peaks corresponding to elements such as Mg, Fe, Sn, Ca, and Al suggest the presence of trace impurities, likely originating from the Ayurvedic Bhasmikaran process, which involves the use of herbal and mineral additives. The detection of Cu and Pt can be attributed to sample preparation and instrument calibration, as these elements are commonly used in EDX analysis. The presence of carbon (C) may be due to organic residues from plant-based components used during synthesis. The relatively high intensity of Zn peaks indicates that ZnO is a dominant phase in the composite, while the Ti peaks confirm the presence of TiO\(_2\). The overall spectrum validates the successful fabrication of a ZnO-TiO\(_2\) composite with potential applications in photocatalysis, biomedical treatments, and antimicrobial activity. The elemental analysis further supports the claim that Jasad Bhasma, when synthesized with TiO\(_2\), retains its nanoscale properties while benefiting from enhanced structural and functional characteristics [75– 77].
Infrared (IR) analysis of TiO\({}_{2}\)-ZnO nanocomposite (Jasad Bhasma) was conducted using a Bruker MultiRAM (Germany) spectrometer to investigate the vibrational modes and functional groups present in the sample. The analysis was performed at room temperature, covering a spectral range of 3600–400 cm\(^{-1}\) with a high resolution of 0.5 cm\(^{-1}\). A Nd-YAG detector was utilized to ensure precise detection of absorption peaks and minimal noise interference.
The obtained IR spectrum displayed characteristic absorption bands corresponding to TiO\({}_{2}\)-ZnO nanoparticles. The most prominent peak was observed at \(\mathrm{\sim}\)442 cm\(^{-1}\), representing the Zn–O stretching vibrations, which confirm the formation of ZnO nanocrystals. Additionally, bands appearing around 3421 cm\(^{-1}\) correspond to O–H stretching, indicating the presence of hydroxyl groups, likely from moisture or plant-based processing materials. Peaks at 2923 cm\(^{-1}\) and 2838 cm\(^{-1}\) are attributed to C–H stretching vibrations, which may originate from organic residues. Figure 5 illustrates the FT-IR spectrum of TiO\({}_{2}\)-ZnO nanocomposite, confirming the successful synthesis of Jasad Bhasma ZnO through the Ayurvedic method with minimal organic contamination [78– 80].
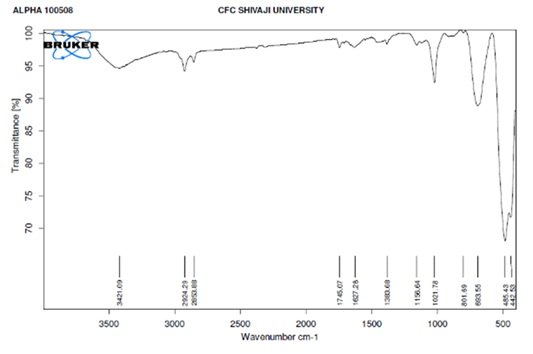
The FT-IR spectrum of the TiO\(_2\)-ZnO (Jasad Bhasma) nanocomposite provides valuable insights into its functional groups and chemical bonding. The broad absorption band around 3421 cm\(^{-1}\) corresponds to the O-H stretching vibration, indicating the presence of hydroxyl groups or adsorbed water molecules, which can play a role in surface reactivity and photocatalytic properties. Peaks observed at 2924 cm\(^{-1}\) and 2853 cm\(^{-1}\) correspond to C-H stretching vibrations, suggesting the presence of residual organic compounds from the Bhasmikaran process. The peak at 1627 cm\(^{-1}\) can be attributed to H-O-H bending vibrations, further confirming adsorbed moisture. The band at 1388 cm\(^{-1}\) is associated with C=O stretching, possibly from residual carbonates. The peaks at 1156 cm\(^{-1}\) and 1021 cm\(^{-1}\) suggest the presence of Zn-OH and Ti-OH stretching vibrations, indicating the formation of hydroxylated metal oxides. The characteristic peaks at lower wavenumbers, specifically at 801 cm\(^{-1}\), 693 cm\(^{-1}\), 485 cm\(^{-1}\), and 442 cm\(^{-1}\), correspond to Zn-O and Ti-O stretching vibrations, confirming the successful formation of a TiO\(_2\)-ZnO composite. The presence of these functional groups indicates strong interactions between ZnO and TiO\(_2\), which can enhance their photocatalytic and biomedical properties. Overall, the FT-IR spectrum confirms the structural integrity and surface modifications of the TiO\(_2\)-ZnO nanocomposite, validating its synthesis and potential applications in antimicrobial and therapeutic applications in traditional medicine [81– 84].
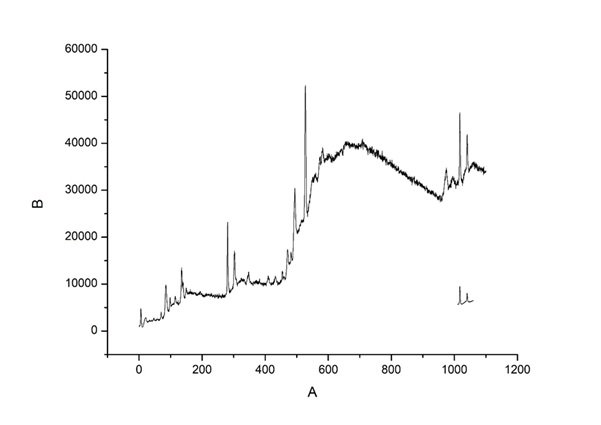
The Raman spectrum of the TiO\(_2\)-ZnO (Jasad Bhasma) nanocomposite provides crucial information about its vibrational modes, phase composition, and structural properties. The observed peaks correspond to the characteristic vibrational modes of both TiO\(_2\) and ZnO, confirming the formation of the nanocomposite. The prominent peaks observed in the range of 100–700 cm\(^{-1}\) can be attributed to the fundamental Raman active modes of TiO\(_2\), specifically the anatase or rutile phases, with strong peaks commonly appearing around 144 cm\(^{-1}\) (Eg), 396 cm\(^{-1}\) (B1g), 513 cm\(^{-1}\) (A1g/B1g), and 638 cm\(^{-1}\) (Eg) for anatase TiO\(_2\). The ZnO component exhibits its characteristic E2 (high) mode around 437 cm\(^{-1}\), which is a signature of the wurtzite phase, confirming its crystalline nature. The presence of additional broad peaks and slight peak shifts suggest strong interactions between TiO\(_2\) and ZnO, which may influence the material’s electronic and phononic properties. The broadening of peaks could also be indicative of nanoscale effects, structural disorder, or defects, which are common in nanocomposite materials. These structural modifications can enhance the optical and photocatalytic properties of the material. The Raman analysis thus confirms the successful formation of the TiO\(_2\)-ZnO nanocomposite with well-defined vibrational features, validating its potential applications in photocatalysis, antimicrobial activity, and biomedical applications rooted in traditional medicine. Figure 6 depicts Raman Spectra of TiO\({}_{2}\)-ZnO nanomaterials [85– 88].
Photoluminescence of TiO\({}_{2}\)-ZnO nanomaterials was conducted using a Bruker MultiRAM (Germany) spectrometer to investigate the vibrational modes and functional groups present in the sample. The analysis was carried out at room temperature, covering a spectral range of 3600–36 cm\(^{-1}\) with a high resolution of 0.5 cm\(^{-1}\). A Nd-YAG detector was employed to ensure precise detection of absorption peaks and minimal noise interference.
The obtained PL spectrum displayed characteristic absorption bands corresponding to TiO\({}_{2}\)-ZnO. The most prominent peak was observed at 300 cm\(^{-1}\), representing the maximum absorbance of the sample. This peak is typically associated with the Zn–O stretching vibrations, confirming the formation of ZnO nanoparticles. Figure 7 reveals the PL Spectrum of TiO\({}_{2}\)-ZnO nanomaterials [89– 96].
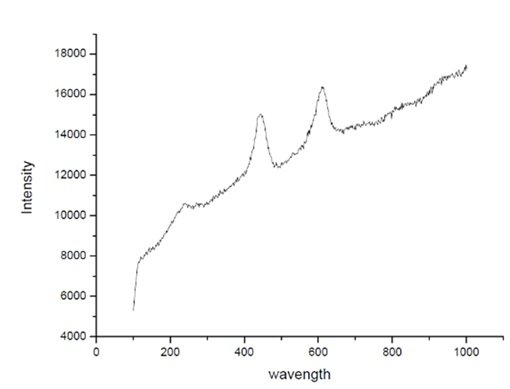
The photoluminescence (PL) spectrum of the TiO\(_2\)-ZnO (Jasad Bhasma) nanocomposite provides insights into its optical properties, defect states, and recombination mechanisms. The spectrum exhibits a strong emission in the UV and visible regions, indicating various electronic transitions. The sharp emission peak in the UV region, typically around 380–420 nm, corresponds to the near-band-edge (NBE) recombination of ZnO and TiO\(_2\), confirming their semiconductor nature. The presence of visible emissions, particularly in the blue, green, or yellow regions, suggests the existence of oxygen vacancies, surface defects, or deep-level states within the nanocomposite. The broad visible emission could be due to defect states arising from Zn interstitials, O vacancies, or Ti\(^{3+}\) states, which play a significant role in charge carrier dynamics.
A reduction in intensity compared to pure ZnO or TiO\(_2\) suggests enhanced charge separation in the composite, reducing recombination and potentially improving photocatalytic performance. The strong interaction between TiO\(_2\) and ZnO enhances charge transfer, suppressing excitonic recombination, which is beneficial for applications in photocatalysis, sensors, and optoelectronics. Overall, the PL spectrum confirms the successful synthesis of the TiO\(_2\)-ZnO nanocomposite with modified optical properties that can be tuned for enhanced functionality [97, 98].
The samples were analyzed using Transmission Electron Microscopy (TEM) on a JEOL ASIA PTE LTD JEM 2100 PLUS, located at CFC, SAIF, Shivaji University, Kolhapur. TEM, which utilizes an electron beam to visualize specimens with greater magnification than conventional optical microscopes, was employed to investigate their microstructure. The microscope was operated with an accelerating voltage of 200 kV, using a combined W and Lab6 filament electron source. Key specifications include a resolution of \(\mathrm{\le}\) 0.23nm and the availability of various imaging and analytical modes such as HRTEM, STEM, EDS, BF, DF, HAADF, SAED, and NBD. This technique yielded detailed information on the samples’ topographical, morphological, compositional, and crystalline characteristics, facilitating structural and textural analysis at the atomic scale [99, 100].
The TEM image of the TiO\(_2\)-ZnO nanocomposite as shown in Figure 8 reveals well-defined nanoparticles with sizes ranging from approximately 24.9 nm to 61.0 nm, exhibiting quasi-spherical or faceted morphology. The observed contrast variations suggest differences in electron density between TiO\(_2\) and ZnO regions, indicating successful composite formation. The presence of layered fringes implies crystallinity and possible heterojunction formation, which can enhance photocatalytic activity. The scale bar (20 nm) and magnifications (446,000x print, 50,000x direct) confirm the nanoscale nature of the material. Such a nanocomposite holds promise for applications in photocatalysis, solar cells, and sensor technology due to improved charge separation and surface interactions.

XPS analysis of a TiO\({}_{2}\)-ZnO nanocomposite confirms the presence of titanium, zinc, and oxygen, indicating the formation of the desired material. The Ti 2p peaks suggest titanium is present, likely as Ti4+ in TiO\({}_{2}\), while the Zn 2p peaks indicate zinc, probably as Zn2+ in ZnO. The O 1s peak provides information about oxygen species within the sample. Analyzing the binding energies and peak areas can reveal the chemical states of the elements, the nanocomposite’s composition, and the nature of the interfacial interactions between TiO\({}_{2}\) and ZnO, though a precise determination requires detailed peak fitting and comparison with reference data [101– 104].
Minimum Inhibitory Concentration (MIC) is the lowest concentration of an antimicrobial agent required to inhibit visible microbial growth. It is a key parameter in antimicrobial susceptibility testing, helping to determine the efficacy of antibiotics, antifungals, and other antimicrobial compounds. MIC values are critical in guiding appropriate drug dosages, monitoring resistance trends, and supporting the development of new therapeutic agents. Standardized methods such as broth dilution, agar dilution, and automated systems (e.g., VITEK, MicroScan) are commonly used for MIC determination.
In many cases, combining antimicrobial agents can enhance their effectiveness, leading to a phenomenon known as synergistic activity. Synergy occurs when the combined effect of two or more antimicrobial agents is greater than the sum of their individual effects. This can be assessed using methods like the checkerboard assay, time-kill curve analysis, and the fractional inhibitory concentration index (FICI). A FICI value \(\mathrm{\le}\)0.5 indicates synergy, while values \(\mathrm{\ge}\)4.0 suggest antagonism. Synergistic combinations are particularly useful in treating multidrug-resistant infections caused by pathogens such as Methicillin-resistant Staphylococcus aureus (MRSA) and Carbapenem-resistant Enterobacteriaceae (CRE). Examples include â-lactam antibiotics combined with â-lactamase inhibitors or azoles combined with echinocandins for fungal infections. Studying MIC and synergistic effects is crucial for optimizing antimicrobial therapy, reducing drug resistance, and improving clinical outcomes. Research in this area can lead to the discovery of novel combination therapies that enhance efficacy while minimizing toxicity and resistance development [105, 108].
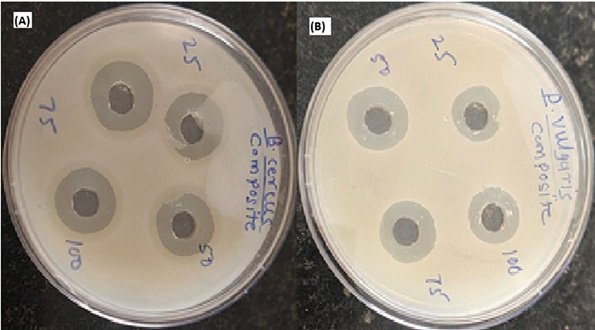
A set of four microorganisms and two fungal strains were used to test the antibacterial and antifungal properties of four distinct samples (two nanoparticle extracts and two composites of that nanoparticle). MIC was used to determine the test samples’ competent inhibition concentration against microorganisms at the primary level. The existence or absence of an inhibitory zone was then qualitatively evaluated.
Titanium dioxide (TiO\(_2\)) and zinc oxide (ZnO) nanocomposites have gained significant attention due to their strong antibacterial properties. These metal oxide nanoparticles exhibit broad-spectrum antimicrobial activity against both Gram-positive and Gram-negative bacteria, including antibiotic-resistant strains. The antibacterial effectiveness of TiO\(_2\)-ZnO nanocomposites is attributed to their ability to generate reactive oxygen species (ROS) under light irradiation, leading to oxidative stress, cell membrane disruption, and bacterial cell death. The synergistic effect of TiO\(_2\) and ZnO enhances their photocatalytic and antibacterial properties. ZnO nanoparticles contribute by generating hydrogen peroxide (H\(_2\)O\(_2\)) and Zn\(^{2+}\) ions, which damage bacterial cells, while TiO\(_2\) nanoparticles enhance ROS production and surface interactions. This combination improves antibacterial efficiency even under visible light conditions. The antibacterial activity of TiO\(_2\)-ZnO nanocomposites is evaluated using standard methods such as disk diffusion, minimum inhibitory concentration (MIC), and bacterial viability assays. Studies have demonstrated that these nanocomposites effectively inhibit bacterial growth and biofilm formation, making them promising candidates for medical coatings, water purification, food packaging, and antibacterial textiles. Additionally, modifying TiO\(_2\)-ZnO nanocomposites with dopants (Ag, Cu), polymer coatings, or surface functionalization can further enhance their antibacterial performance and stability. However, concerns regarding nanoparticle toxicity and environmental impact require further research to ensure their safe and effective application. Overall, TiO\(_2\)-ZnO nanocomposites present a promising approach for developing next-generation antibacterial materials to combat microbial infections and antibiotic resistance [109– 114].
Titanium dioxide (TiO\(_2\)) and zinc oxide (ZnO) nanocomposites have shown significant potential as antifungal agents due to their strong photocatalytic and antimicrobial properties. These nanocomposites exhibit broad-spectrum antifungal activity against pathogenic fungi such as Candida albicans, Aspergillus niger, and Cryptococcus neoformans. Their antifungal mechanism primarily involves the generation of reactive oxygen species (ROS), which induce oxidative stress, damage fungal cell membranes, and disrupt cellular functions, ultimately leading to fungal cell death.
The synergistic interaction between TiO\(_2\) and ZnO enhances their antifungal efficacy. ZnO nanoparticles contribute by releasing Zn\(^{2+}\) ions, which interfere with fungal metabolism and cell integrity, while TiO\(_2\) nanoparticles amplify ROS production and improve photocatalytic performance. This combination enables effective antifungal action under UV and visible light exposure, making these nanocomposites ideal for antifungal coatings and biomedical applications. The antifungal activity of TiO\(_2\)-ZnO nanocomposites is assessed using standard techniques such as disk diffusion, minimum inhibitory concentration (MIC), and fungal viability assays. Studies have demonstrated their ability to inhibit fungal growth, biofilm formation, and spore germination, making them promising for medical devices, wound dressings, food packaging, and water purification systems.
Further modifications, such as doping with Ag or Cu, surface functionalization, and polymer coatings, can enhance their antifungal efficiency and biocompatibility. However, potential toxicity and environmental impact require further investigation to ensure safe applications. Thus, TiO\(_2\)-ZnO nanocomposites offer a promising alternative to conventional antifungal agents, helping to combat fungal infections and resistance. Figure 9 shows Antimicrobial Activity of TiO\({}_{2}\)– ZnO Sample [ 115– 118].
| Crease Recovery Angle | ||||
| Reading | Warp | Weft | ||
| control | TiO\(_2\)-ZnO Padded Fabric | control | TiO\(_2\)-ZnO Padded Fabric | |
| 1 | 63 | 80 | 40 | 80 |
| 2 | 61 | 80 | 55 | 100 |
| 3 | 40 | 82 | 60 | 60 |
| 4 | 55 | 80 | 55 | 70 |
| 5 | 51 | 90 | 55 | 100 |
| Mean | 54 | 82.4 | 53 | 82 |
This bar graph illustrates the crease recovery angle of a titanium dioxide-zinc oxide (TiO\({}_{2}\)-ZnO) nanocomposite padded fabric in the warp direction, comparing it to a control sample across five trials. The data consistently demonstrates a significant increase in crease recovery angle for the TiO\({}_{2}\)-ZnO-padded fabric compared to the control in each trial, indicating enhanced crease resistance. Notably, trials 1, 2, 4, and 5 show a substantial increase in crease recovery with the nanocomposite treatment, while trial 3 also shows improvement, albeit to a lesser degree. This consistent enhancement across all trials suggests that the TiO\({}_{2}\)-ZnO nanocomposite padding effectively improves the fabric’s ability to recover from creases in the warp direction. Although there’s some variation in the magnitude of improvement across trials, it’s evident that the nanocomposite treatment positively impacts the fabric’s crease recovery. This improvement likely stems from the reinforcement of the fabric structure by the TiO\({}_{2}\)-ZnO nanocomposite, which reduces deformation and promotes quicker recovery. The observed differences in improvement magnitude across trials might be due to variations in fabric composition, padding consistency, or testing conditions. Overall, the graph strongly supports the conclusion that TiO\({}_{2}\)-ZnO nanocomposite padding significantly enhances the crease recovery angle of the fabric in the warp direction, indicating improved crease resistance. Figure 10 represents Crease Recovery Angle of TiO\({}_{2}\)-ZnO padded fabric Warp. Table 1 depicts Crease Recovery Angle of TiO\({}_{2}\)-ZnO Padded Fabric [119– 120].

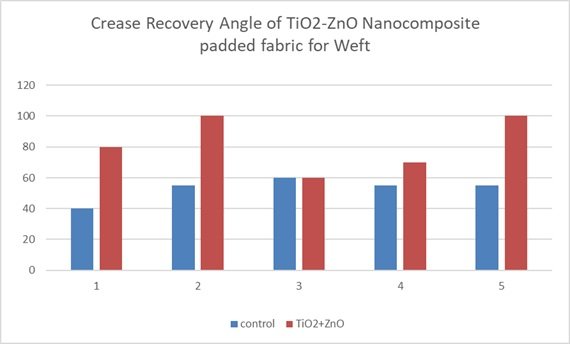
This bar graph as shown in Figure 11 illustrates the crease recovery angle of a titanium dioxide-zinc oxide (TiO\(_2\)-ZnO) nanocomposite padded fabric in the weft direction, comparing it to a control sample across five trials. The data consistently demonstrates a significant increase in crease recovery angle for the TiO\({}_{2}\)-ZnO-padded fabric compared to the control in each trial, indicating enhanced crease resistance.
| Stiffness (in cm) | ||||
| Reading | Warp | Weft | ||
| control | TiO\(_2\)-ZnO Padded Fabric | control | TiO\(_2\)-ZnO Padded Fabric | |
| 1 | 2.65 | 2.4 | 1.6 | 2 |
| 2 | 2.9 | 2.2 | 1.6 | 1.9 |
| 3 | 3 | 2.6 | 1.8 | 1.9 |
| 4 | 2.8 | 2.1 | 1.9 | 1.7 |
| 5 | 2.4 | 2.8 | 1.6 | 1.7 |
| 6 | 2.6 | 2.2 | 1.7 | 1.9 |
| 7 | 2.6 | 2.5 | 1.6 | 1.8 |
| 8 | 2.8 | 2.7 | 2 | 2 |
| 9 | 2.6 | 2.5 | 1.7 | 1.6 |
| 10 | 2.6 | 2.4 | 1.7 | 2 |
| 11 | 2.6 | 2.1 | 1.5 | 1.7 |
| 12 | 2.4 | 2.9 | 1.8 | 2 |
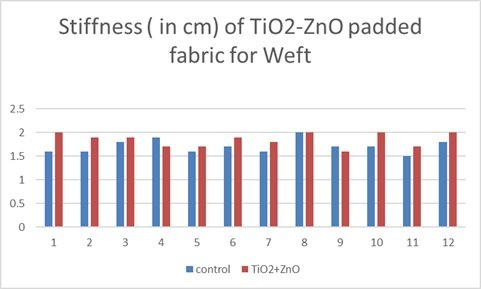
| Mean | 2.6625 | 2.45 | 1.708333 | 1.85 |
This bar graph illustrates the stiffness, measured in centimeters, of a titanium dioxide-zinc oxide (TiO\(_2\)-ZnO) nanocomposite padded fabric in the warp direction, comparing it to a control sample across twelve trials. The data reveals a consistent trend: The TiO\({}_{2}\)-ZnO-padded fabric exhibits lower stiffness compared to the control in nearly all trials, indicating a softening effect from the nanocomposite treatment. Notably, trials 1, 2, 3, 4, 6, 7, 8, 9, 10, and 11 show a more pronounced reduction in stiffness with the nanocomposite padding, while trials 5 and 12 indicate a smaller, though still present, decrease. This consistent softening effect suggests that the TiO\({}_{2}\)-ZnO nanocomposite alters the fabric’s structure or fiber interactions, leading to increased flexibility. Although the degree of stiffness reduction varies across trials, possibly due to inconsistencies in the padding process or fabric variations, the overall trend is clear. The consistent decrease in stiffness with TiO\({}_{2}\)-ZnO padding suggests that this treatment could be beneficial for applications where fabric softness and flexibility are desired. The graph strongly supports the conclusion that TiO\(_2\)-ZnO padding reduces the stiffness of the fabric in the warp direction, highlighting its potential to enhance fabric handle and drape. Figure 12 shows Stiffness of TiO\({}_{2}\)-ZnO padded fabric Warp and Table 2 depicts Stiffness for TiO\({}_{2}\)-ZnO Nano-composite Padded Fabric [121].
This bar graph as shown in Figure 13 illustrates the stiffness, measured in centimeters, of a titanium dioxide-zinc oxide (TiO\({}_{2}\)-ZnO) nanocomposite padded fabric in the weft direction, comparing it to a control sample across twelve trials. The data consistently demonstrates a decrease in stiffness for the TiO\({}_{2}\)-ZnO-padded fabric compared to the control in nearly all trials, indicating a softening effect from the nanocomposite treatment.

Notably, trials 1, 2, 3, 4, 6, 7, 8, 9, 10, and 12 show a more pronounced reduction in stiffness with the nanocomposite padding, while trials 5 and 11 indicate a smaller, though still present, decrease. This consistent softening effect suggests that the TiO\({}_{2}\)-ZnO nanocomposite alters the fabric’s structure or fiber interactions, leading to increased flexibility. Although the degree of stiffness reduction varies across trials, possibly due to inconsistencies in the padding process or fabric variations, the overall trend is clear. The consistent decrease in stiffness with TiO\({}_{2}\)-ZnO padding suggests that this treatment could be beneficial for applications where fabric softness and flexibility are desired. The graph strongly supports the conclusion that TiO\({}_{2}\)-ZnO padding reduces the stiffness of the fabric in the weft direction, highlighting its potential to enhance fabric handle and drape.
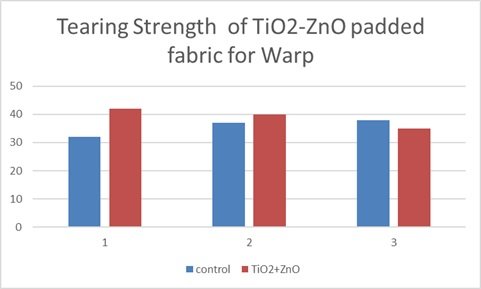
This bar graph illustrates the tearing strength of a titanium dioxide-zinc oxide (TiO\({}_{2}\)-ZnO) nanocomposite padded fabric in the warp direction, comparing it to a control sample across three trials. The data presents a mixed trend regarding the impact of the TiO\({}_{2}\)-ZnO padding on tearing strength. Notably, trial 1 shows a significant increase in tearing strength for the nanocomposite-padded fabric compared to the control, suggesting enhanced tear resistance. However, trials 2 and 3 exhibit a decrease in tearing strength with the nanocomposite treatment, indicating a potential weakening effect. This inconsistency across trials suggests that the TiO\({}_{2}\)-ZnO nanocomposite padding does not consistently improve the fabric’s resistance to tearing in the warp direction. The observed differences in tearing strength may be attributed to variations in fabric composition, padding consistency, or testing conditions. It is also possible that the specific interaction between the TiO\({}_{2}\)-ZnO nanocomposite and the fabric structure in the warp direction is complex, leading to both strengthening and weakening effects depending on the trial. Further investigation with a larger sample size and controlled conditions is needed to determine the precise impact of TiO\({}_{2}\)-ZnO nanocomposite padding on the tearing strength of fabric in the warp direction. Overall, the graph indicates that the effect of TiO\({}_{2}\)-ZnO padding on tearing strength in the warp direction is not uniformly positive and requires further study. Figure 14 shows Tearing Strength of TiO\({}_{2}\)-ZnO padded fabric Warp. Table 3 depicts Tearing strength for TiO\({}_{2}\)-ZnO Padded Fabric [122– 124].
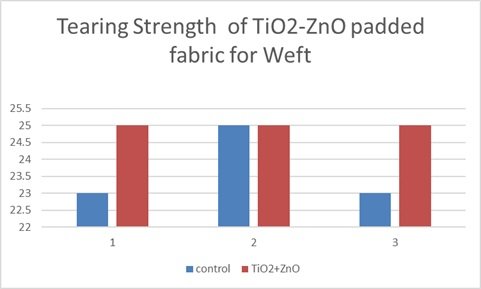
This bar graph as shown in Figure 15 illustrates the tearing strength of a titanium dioxide-zinc oxide (TiO\({}_{2}\)-ZnO) nanocomposite padded fabric in the weft direction, comparing it to a control sample across three trials. The data consistently demonstrates an increase in tearing strength for the TiO\({}_{2}\)-ZnO-padded fabric compared to the control in each trial, indicating improved tear resistance. Notably, trials 1 and 3 show a more pronounced increase in tearing strength with the nanocomposite treatment, while trial 2 shows a smaller, though still present, enhancement. This consistent trend across all trials suggests that the TiO\({}_{2}\)-ZnO nanocomposite padding effectively improves the fabric’s resistance to tearing in the weft direction. Although there’s some variation in the magnitude of improvement across trials, it’s evident that the nanocomposite treatment positively impacts the fabric’s tearing strength. This improvement likely stems from the reinforcement of the fabric structure by the TiO\({}_{2}\)-ZnO nanocomposite, which enhances the fabric’s ability to withstand tearing forces. The observed differences in improvement magnitude across trials might be due to variations in fabric composition, padding consistency, or testing conditions. Overall, the graph strongly supports the conclusion that TiO\({}_{2}\)-ZnO nanocomposite padding significantly enhances the tearing strength of the fabric in the weft direction, indicating improved durability.
This paper detailed the synthesis, characterization, and applications of TiO\({}_{2}\)-ZnO nanocomposites, focusing on their enhanced antimicrobial properties and potential in textile applications. The study successfully synthesized the nanocomposites and characterized them using various techniques, including DRS-UV-visible, XRD, SEM, EDX, FT-IR, Raman spectroscopy, and photoluminescence. The application of the nanocomposites to textiles was evaluated through antimicrobial activity tests and assessments of crease recovery, stiffness, and tearing strength, demonstrating their potential to impart antimicrobial properties and enhance fabric performance.
There are no conflicts of interest to declare.
The authors pay sincere tribute to Late Ms Deepika Rai Dhirendra Prasad who suddenly left this world and lived very short span of life, we the authors remember her on this occasion and pray Almighty God for peace of her holy soul. Her sweet memories would always be in the heart of authors. The authors are thankful to Mrs. Archana Kanthe, Jaysingpur College, Jaysingpur, India for providing certain characterizations.
Narayanan, K. B., & Sakthivel, N. (2010). Biological synthesis of metal nanoparticles by microbes. Advances in Colloid and Interface Science, 156(1-2), 1-13.
Baig, N., Kammakakam, I., & Falath, W. (2021). Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Materials Advances, 2(6), 1821-1871.
Prasad, R. D., Desai, C. B., Srivastava, O. P., Prasad, S. R., Bhat, T. S., Kamble, B., … & Prasad, R. Y. (2023). A critical review on recent developments in advanced supercapacitors for veterinary medicine. ES Food & Agroforestry, 11, 805.
Prasad, S. R., Kumbhar, V. B., & Prasad, N. R. (2023). Applications of nanotechnology in textile: A review. ES Food & Agroforestry, 15, 1019.
Gaur, M., Misra, C., Yadav, A. B., Swaroop, S., Maolmhuaidh, F. Ó., Bechelany, M., & Barhoum, A. (2021). Biomedical applications of carbon nanomaterials: fullerenes, quantum dots, nanotubes, nanofibers, and graphene. Materials, 14(20), 5978.
Prasad, R. D., Sahoo, A. K., Shrivastav, O. P., Charmode, N., Prasad, S. R., Kamat, R., … & Prasad, N. R. (2022). A review on aspects of nanotechnology in food science and animal nutrition. ES Food & Agroforestry, 8, 12-46.
Barhoum, A., Pal, K., Rahier, H., Uludag, H., Kim, I. S., & Bechelany,9 M. (2019). Nanofibers as new-generation materials: From spinning and nano-spinning fabrication techniques to emerging applications. Applied Materials Today, 17, 1-35.
Miyazaki, K., & Islam, N. (2007). Nanotechnology systems of innovation—An analysis of industry and academia research activities. Technovation, 27(11), 661-675.
Prasad, R. D., Teli, B., Prasad, R. S., Prasad, R. B., Prasad, S. R., Sinha, P., … & Guo, Z. (2024). A review on thin film technology and nanomaterial characterization techniques. ES Materials & Manufacturing, 25, 1198.
Constable, E. C. (2019). Evolution and understanding of the d-block elements in the periodic table. Dalton Transactions, 48(26), 9408-9421.
Prasad, R. D., Sarvalkar, P. D., Prasad, N., Prasad, R. S., Prasad, R. B., Prasad, R. R., … & Guo, Z. (2024). Emerging trends of bioactive nano-materials in modern veterinary science and animal husbandry. ES Food & Agroforestry, 18, 1144.
Jha, D. K., Samrat, R., & Sanyal, P. (2021). The first evidence of controlled use of fire by prehistoric humans during the Middle Paleolithic phase from the Indian subcontinent. Palaeogeography, Palaeoclimatology, Palaeoecology, 562, 110151.
Bayda, S., Adeel, M., Tuccinardi, T., Cordani, M., & Rizzolio, F. (2019). The history of nanoscience and nanotechnology: from chemical–physical applications to nanomedicine. Molecules, 25(1), 112.
Anselmo, A. C., & Mitragotri, S. (2019). Nanoparticles in the clinic: An update. Bioengineering & Translational Medicine, 4(3), e10143.
Prasad, R. D., Prasad, R. S., Prasad, R. B., Prasad, S. R., Singha, S. B., Singha, D., … & Navathe, G. J. (2024). A review on modern characterization techniques for analysis of nanomaterials and biomaterials. ES Energy & Environment, 23, 1087.
Ibrahim Khan, K. S., & Khan, I. (2017). Nanoparticles: Properties, applications and toxicities. Arabian Journal of Chemistry, 12(7), 908-931.
Prasad, R. D., Prasad, S. R., Shrivastav, O. P., Kanthe, A. R., Waghmare, S. R., Shaikh, V. S., … & Shaikh, Y. I. (2023). Biogenic synthesis of nano-silver and their anti-microbial efficacy. ES Food & Agroforestry, 11(2), 836.
Prasad, R. D., Prasad, N. R., Prasad, R. S., Prasad, N., Prasad, S. R., Shrivastav, M., … & Kamble, J. (2024). A review on nanotechnology from prehistoric to modern age. ES General, 4, 1117.
Harish, V., Ansari, M. M., Tewari, D., Gaur, M., Yadav, A. B., García-Betancourt, M. L., … & Barhoum, A. (2022). Nanoparticle and nanostructure synthesis and controlled growth methods. Nanomaterials, 12(18), 3226.
Prasad, R. D., Prasad, R. J., Shrivastav, R. K., Charmode, N., Mamidpelliwar, P. M., Shrivastav, O. P., … & Sarvalkar, P. (2023). A review on concept of veterinary bio-technology and livestock products in medicine. ES Food & Agroforestry, 14, 1004.
Fendler, J. H. (Ed.). (2008). Nanoparticles and Nanostructured Films: Preparation, Characterization, and Applications. John Wiley & Sons.
Jeevanandam, J., Barhoum, A., Chan, Y. S., Dufresne, A., & Danquah, M. K. (2018). Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. Beilstein Journal of Nanotechnology, 9(1), 1050-1074.
Patil, R. M., Deshpande, P. P., Aalhate, M., Gananadhamu, S., & Singh, P. K. (2022). An update on sophisticated and advanced analytical tools for surface characterization of nanoparticles. Surfaces and Interfaces, 33, 102165.
Prasad, R. D., Charmode, N., Shrivastav, O. P., Prasad, S. R., Moghe, A., Sarvalkar, P. D., & Prasad, N. R. (2021). A review on concept of nanotechnology in veterinary medicine. ES Food & Agroforestry, 4(5), 28-60.
Shi, J., Votruba, A. R., Farokhzad, O. C., & Langer, R. (2010). Nanotechnology in drug delivery and tissue engineering: from discovery to applications. Nano Letters, 10(9), 3223-3230.
Di Maria, F., Lodola, F., Zucchetti, E., Benfenati, F., & Lanzani, G. (2018). The evolution of artificial light actuators in living systems: from planar to nanostructured interfaces. Chemical Society Reviews, 47(13), 4757-4780.
Prasad, R. D., Desai, C. B., Shrivastav, O. P., Charmode, N., Prasad, S. R., Samant, A., … & Sarvalkar, P. (2022). A critical review on design and development of carbonaceous materials for veterinary medicine. ES Food & Agroforestry, 9, 15-38.
Padvi, M. N., Moholkar, A. V., Prasad, S. R., & Prasad, N. R. (2020). A critical review on design and development of gas sensing materials. ES Engineered Science, 15, 20-37.
Sai-Halasz, G. A., Wordeman, M. R., Kern, D. P., Rishton, S. A., Ganin, E., Chang, T. H. P., & Dennard, R. H. (1990). Experimental technology and performance of 0.1-µm-gate-length FETs operated at liquid-nitrogen temperature. IBM Journal of Research and Development, 34(4), 452-465.
Pradeep, T. (2009). Noble metal nanoparticles for water purification: A critical review. Thin Solid Films, 517(24), 6441-6478.
Prasad, R. D., Prasad, N. R., Prasad, R. S., Prasad, N., Prasad, S. R., Shrivastav, M., … & Kamble, J. (2024). A review on nanotechnology from prehistoric to modern age. ES General, 4, 1117.
Patel, K. D., Singh, R. K., & Kim, H. W. (2019). Carbon-based nanomaterials as an emerging platform for theranostics. Materials Horizons, 6(3), 434-469.
Griffin, S., Masood, M. I., Nasim, M. J., Sarfraz, M., Ebokaiwe, A. P., Schäfer, K. H., … & Jacob, C. (2017). Natural nanoparticles: a particular matter inspired by nature. Antioxidants, 7(1), 3.
Ciftcioglu, N., McKay, D. S., Mathew, G., & Kajander, O. E. (2006). Nanobacteria: Fact or fiction? characteristics, detection, and medical importance of novel self-replicating, calcifying nanoparticles, Journal of Investigative Medicine, 54, 385-394.
Prasad, R. D., Desai, C. B., Shrivastav, O. P., Charmode, N., Prasad, S. R., Samant, A., … & Sarvalkar, P. (2022). A critical review on design and development of carbonaceous materials for veterinary medicine. ES Food & Agroforestry, 9, 15-38.
Prasad, R. D., Karvekar, O. S., Pawar, P. B., Charmode, N., Shrivastav, O. P., Prasad, S. R., … & Prasad, N. R. (2021). A review on nanotechnological aspects in veterinary medicine. Research Journal of Life Sciences, Bioinformatics, Pharmaceuticals and Chemical Sciences, 7, 42-85.
Gaur, M., Misra, C., Yadav, A. B., Swaroop, S., Maolmhuaidh, F. Ó., Bechelany, M., & Barhoum, A. (2021). Biomedical applications of carbon nanomaterials: fullerenes, quantum dots, nanotubes, nanofibers, and graphene. Materials, 14(20), 5978.
Prasad, R. D., Prasad, N. R., Prasad, R. B., Prasad, R. Y., Prasad, S. R., Gour, M., … & Sarvalkar, P. (2023). A critical review on bio-mimetic synthesis of transition metal nanoparticles: their biomedical applications in veterinary medicine. ES Food & Agroforestry, 15, 1027.
Marques, A. C., Vale, M., Vicente, D., Schreck, M., Tervoort, E., & Niederberger, M. (2021). Porous silica microspheres with immobilized titania nanoparticles for in‐flow solar‐driven purification of wastewater. Global Challenges, 5(5), 2000116.
Sharifi, S., Behzadi, S., Laurent, S., Forrest, M. L., Stroeve, P., & Mahmoudi, M. (2012). Toxicity of nanomaterials. Chemical Society Reviews, 41(6), 2323-2343.
Rambaran, T., & Schirhagl, R. (2022). Nanotechnology from lab to industry–a look at current trends. Nanoscale Advances, 4(18), 3664-3675.
Prasad, R. D., Prasad, R. S., Prasad, R. B., Prasad, S. R., Singha, S. B., Singha, D., … & Navathe, G. J. (2024). A review on modern characterization techniques for analysis of nanomaterials and biomaterials. ES Energy & Environment, 23, 1087.
Sadik, O. (2017). The two faces of nanotechnology. American Scientist, 105, 208.
Ravichandran, R. (2010). Nanotechnology applications in food and food processing: innovative green approaches, opportunities and uncertainties for global market. International Journal of Green Nanotechnology: Physics and Chemistry, 1(2), P72-P96.
Chandra, B. P., Chandra, V. K., & Jha, P. (2015). Luminescence of II-VI semiconductor nanoparticles. Solid State Phenomena, 222, 1-65.
Binnig, G., Rohrer, H., Gerber, C., & Weibel, E. (1982). Surface studies by scanning tunneling microscopy. Physical Review Letters, 49(1), 57-60.
Binnig, G., Quate, C. F., & Gerber, C. (1986). Atomic force microscope. Physical Review Letters, 56(9), 930-933.
Butt, H. J., Cappella, B., & Kappl, M. (2005). Force measurements with the atomic force microscope: Technique, interpretation and applications. Surface Science Reports, 59(1-6), 1-152.
Prasad, R. D., Prasad, R. S., Shaikh, Y. I., Prasad, S. R., Padvi, M. N., Sarvalkar, P. D., … & Patil, P. D. (2024). A critical review on uses of gases in veterinary medicine and gas sensing materials. ES Materials & Manufacturing, 23, 1084.
Makwana, B. A., Vyas, D. J., Bhatt, K. D., Jain, V. K., & Agrawal, Y. K. (2015). Highly stable antibacterial silver nanoparticles as selective fluorescent sensor for Fe3+ ions. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 134, 73-80.
Gawande, M. B., Goswami, A., Asefa, T., Guo, H., Biradar, A. V., Peng, D. L., … & Varma, R. S. (2015). Core–shell nanoparticles: synthesis and applications in catalysis and electrocatalysis. Chemical Society Reviews, 44(21), 7540-7590.
Schaming, D., & Remita, H. (2015). Nanotechnology: from the ancient time to nowadays. Foundations of Chemistry, 17, 187-205.
Mie, G. (1908). Beiträge zur Optik trüber Medien, speziell kolloidaler Metallösungen. Annalen Der Physik, 330(3), 377-445.
Kelly, K. L., Coronado, E., Zhao, L. L., & Schatz, G. C. (2003). The optical properties of metal nanoparticles: The influence of size, shape, and dielectric environment. The Journal of Physical Chemistry B, 107(3), 668-677.
Gou, L., Chipara, M., & Zaleski, J. M. (2007). Convenient, rapid synthesis of Ag nanowires. Chemistry of Materials, 19(7), 1755-1760.
Chen, X., & Mao, S. S. (2007). Titanium dioxide nanomaterials: synthesis, properties, modifications, and applications. Chemical Reviews, 107(7), 2891-2959.
Joudeh, N., & Linke, D. (2022). Nanoparticle classification, physicochemical properties, characterization, and applications: a comprehensive review for biologists. Journal of Nanobiotechnology, 20(1), 262.
Ikhmayies, S. J. (2014). Characterization of nanomaterials. JOM, 66(1), 28-29.
Geoffrion, L. D., & Guisbiers, G. (2020). Quantum confinement: Size on the grill!. Journal of Physics and Chemistry of Solids, 140, 109320.
Kolahalam, L. A., Viswanath, I. K., Diwakar, B. S., Govindh, B., Reddy, V., & Murthy, Y. L. N. (2019). Review on nanomaterials: Synthesis and applications. Materials Today: Proceedings, 18, 2182-2190.
Mekuye, B., & Abera, B. (2023). Nanomaterials: An overview of synthesis, classification, characterization, and applications. Nano Select, 4(8), 486-501.
Jadhav, P., Shinde, S., Suryawanshi, S. S., Teli, B., Patil, P. S., Ramteke, A. A., … & Prasad, N. R. (2020). Green AgNPs decorated ZnO nanocomposites for dye degradation and antimicrobial applications. Engineered Science, 12(21), 79-94.
Cho, G., Park, Y., Hong, Y. K., & Ha, D. H. (2019). Ion exchange: an advanced synthetic method for complex nanoparticles. Nano Convergence, 6, 1-17.
Buzea, C., Pacheco, I. I., & Robbie, K. (2007). Nanomaterials and nanoparticles: sources and toxicity. Biointerphases, 2(4), MR17-MR71.
Kolahalam, L. A., Viswanath, I. K., Diwakar, B. S., Govindh, B., Reddy, V., & Murthy, Y. L. N. (2019). Review on nanomaterials: Synthesis and applications. Materials Today: Proceedings, 18, 2182-2190.
Atanasov, A. G., Zotchev, S. B., Dirsch, V. M., & Supuran, C. T. (2021). Natural products in drug discovery: advances and opportunities. Nature Reviews Drug Discovery, 20(3), 200-216.
Thomford, N. E., Senthebane, D. A., Rowe, A., Munro, D., Seele, P., Maroyi, A., & Dzobo, K. (2018). Natural products for drug discovery in the 21st century: innovations for novel drug discovery. International Journal of Molecular Sciences, 19(6), 1578.
Khan, R. A. (2018). Natural products chemistry: The emerging trends and prospective goals. Saudi Pharmaceutical Journal, 26(5), 739-753.
Virlan, M. J. R., Miricescu, D., Radulescu, R., Sabliov, C. M., Totan, A., Calenic, B., & Greabu, M. (2016). Organic nanomaterials and their applications in the treatment of oral diseases. Molecules, 21(2), 207.
Shrestha, S., Diogenes, A., & Kishen, A. (2015). Temporal-controlled dexamethasone releasing chitosan nanoparticle system enhances odontogenic differentiation of stem cells from apical papilla. Journal of Endodontics, 41(8), 1253-1258.
Yanat, M., & Schroën, K. (2021). Preparation methods and applications of chitosan nanoparticles; with an outlook toward reinforcement of biodegradable packaging. Reactive and Functional Polymers, 161, 104849.
Chronopoulou, L., Nocca, G., Castagnola, M., Paludetti, G., Ortaggi, G., Sciubba, F., … & Palocci, C. (2016). Chitosan based nanoparticles functionalized with peptidomimetic derivatives for oral drug delivery. New Biotechnology, 33(1), 23-31.
Toshima, N., & Yonezawa, T. (1998). Bimetallic nanoparticles—novel materials for chemical and physical applications. New Journal of Chemistry, 22(11), 1179-1201.
Nascimento, M. A., Cruz, J. C., Rodrigues, G. D., de Oliveira, A. F., & Lopes, R. P. (2018). Synthesis of polymetallic nanoparticles from spent lithium-ion batteries and application in the removal of reactive blue 4 dye. Journal of Cleaner Production, 202, 264-272.
Ealia, S. A. M., & Saravanakumar, M. P. (2017, November). A review on the classification, characterisation, synthesis of nanoparticles and their application. In Iop Conference Series: Materials Science and Engineering (Vol. 263, No. 3, p. 032019). IOP Publishing.
Mody, V. V., Siwale, R., Singh, A., & Mody, H. R. (2010). Introduction to metallic nanoparticles. Journal of Pharmacy and Bioallied Sciences, 2(4), 282-289.
Chandrakala, V., Aruna, V., & Angajala, G. (2022). Review on metal nanoparticles as nanocarriers: Current challenges and perspectives in drug delivery systems. Emergent Materials, 5(6), 1593-1615.
Dreaden, E. C., Alkilany, A. M., Huang, X., Murphy, C. J., & El-Sayed, M. A. (2012). The golden age: gold nanoparticles for biomedicine. Chemical Society Reviews, 41(7), 2740-2779.
Suresh, S. (2013). Semiconductor nanomaterials, methods and applications: a review. Nanoscience and Nanotechnology, 3(3), 62-74.
Terna, A. D., Elemike, E. E., Mbonu, J. I., Osafile, O. E., & Ezeani, R. O. (2021). The future of semiconductors nanoparticles: Synthesis, properties and applications. Materials Science and Engineering: B, 272, 115363.
Vinyas, M., Athul, S. J., Harursampath, D., Loja, M., & Thoi, T. N. (2019). A comprehensive review on analysis of nanocomposites: from manufacturing to properties characterization. Materials Research Express, 6(9), 092002.
Sarvalkar, P. D., Mandavkar, R. R., Nimbalkar, M. S., Sharma, K. K., Patil, P. S., Kamble, G. S., & Prasad, N. R. (2021). Bio-mimetic synthesis of catalytically active nano-silver using Bos taurus (A-2) urine. Scientific Reports, 11(1), 16934.
Kardani, A., & Montazeri, A. (2020). Metal-matrix nanocomposites under compressive loading: Towards an understanding of how twinning formation can enhance their plastic deformation. Scientific Reports, 10(1), 9745.
Shameem, M. M., Sasikanth, S. M., Annamalai, R., & Raman, R. G. (2021). A brief review on polymer nanocomposites and its applications. Materials Today: Proceedings, 45, 2536-2539.
Shukla, P., & Saxena, P. (2021). Polymer nanocomposites in sensor applications: a review on present trends and future scope. Chinese Journal of Polymer Science, 39(6), 665-691.
Pietrzykowska, E., Romelczyk-Baishya, B., Wojnarowicz, J., Sokolova, M., Szlazak, K., Swieszkowski, W., … & Lojkowski, W. (2020). Preparation of a ceramic matrix composite made of hydroxyapatite nanoparticles and polylactic acid by consolidation of composite granules. Nanomaterials, 10(6), 1060.
Palmero, P. (2015). Structural ceramic nanocomposites: a review of properties and powders’ synthesis methods. Nanomaterials, 5(2), 656-696.
Qureshi, M. Z. A., Bilal, S., Malik, M. Y., Raza, Q., Sherif, E. S. M., & Li, Y. M. (2021). Dispersion of metallic/ceramic matrix nanocomposite material through porous surfaces in magnetized hybrid nanofluids flow with shape and size effects. Scientific Reports, 11(1), 12271.
Nazeruddin, G. M., Prasad, S. R., Shaikh, Y. I., Ansari, J., Sonawane, K. D., Nayak, A. K., & Prasad, N. R. (2016). In-vitro Bio-fabrication of Multi-applicative Silver Nanoparticles using Nicotiana tabacum leaf extract. Life Science Informatics Publications, 2(4), 6-30.
Maiti, D., Tong, X., Mou, X., & Yang, K. (2019). Carbon-based nanomaterials for biomedical applications: a recent study. Frontiers in Pharmacology, 9, 1401.
Mahor, A., Singh, P. P., Bharadwaj, P., Sharma, N., Yadav, S., Rosenholm, J. M., & Bansal, K. K. (2021). Carbon-Based Nanomaterials for Delivery of Biologicals and Therapeutics: A Cutting-Edge Technology. C, 7(1), 19.
Patel, K. D., Singh, R. K., & Kim, H. W. (2019). Carbon-based nanomaterials as an emerging platform for theranostics. Materials Horizons, 6(3), 434-469.
Abbasi, E., Aval, S. F., Akbarzadeh, A., Milani, M., Nasrabadi, H. T., Joo, S. W., … & Pashaei-Asl, R. (2014). Dendrimers: synthesis, applications, and properties. Nanoscale Research Letters, 9, 1-10.
Mittal, P., Saharan, A., Verma, R., Altalbawy, F. M., Alfaidi, M. A., Batiha, G. E. S., … & Rahman, M. S. (2021). Dendrimers: a new race of pharmaceutical nanocarriers. BioMed Research International, 2021(1), 8844030.
Nizami, M. Z. I., Xu, V. W., Yin, I. X., Lung, C. Y. K., Niu, J. Y., & Chu, C. H. (2022). Ceramic nanomaterials in caries prevention: a narrative review. Nanomaterials, 12(24), 4416.
Al‐Harbi, F. A., Ayad, N. M., ArRejaie, A. S., Bahgat, H. A., & Baba, N. Z. (2017). Effect of aging regimens on resin nanoceramic chairside CAD/CAM material. Journal of Prosthodontics, 26(5), 432-439.
Tang, Y., Yu, M., Zhang, Z., Chen, J., Xiang, H., Xing, X., & Fang, L. (2020). A novel tungstate Li3Nd3W2O12 with garnet structure for low-temperature cofired ceramic technology. Journal of the European Ceramic Society, 40(4), 1386-1389.
Jamkhande, P. G., Ghule, N. W., Bamer, A. H., & Kalaskar, M. G. (2019). Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. Journal of Drug Delivery Science and Technology, 53, 101174.
Zhao, R., Xiang, J., Wang, B., Chen, L., & Tan, S. (2022). Recent advances in the development of noble metal NPs for cancer therapy. Bioinorganic Chemistry and Applications, 2022(1), 2444516.
Isaac, N. A., Pikaar, I., & Biskos, G. (2022). Metal oxide semiconducting nanomaterials for air quality gas sensors: operating principles, performance, and synthesis techniques. Microchimica Acta, 189(5), 196.
Dascalu, I., Somacescu, S., Hornoiu, C., Calderon-Moreno, J. M., Stanica, N., Stroescu, H., … & Gartner, M. (2018). Sol-gel Zn, Fe modified SnO2 powders for CO sensors and magnetic applications. Process Safety and Environmental Protection, 117, 722-729.
Mehta, M., Bui, T. A., Yang, X., Aksoy, Y., Goldys, E. M., & Deng, W. (2023). Lipid-based nanoparticles for drug/gene delivery: An overview of the production techniques and difficulties encountered in their industrial development. ACS Materials Au, 3(6), 600-619.
Zielińska, A., Carreiró, F., Oliveira, A. M., Neves, A., Pires, B., Venkatesh, D. N., … & Souto, E. B. (2020). Polymeric nanoparticles: production, characterization, toxicology and ecotoxicology. Molecules, 25(16), 3731.
Xiao, X., Teng, F., Shi, C., Chen, J., Wu, S., Wang, B., … & Li, W. (2022). Polymeric nanoparticles—promising carriers for cancer therapy. Frontiers in Bioengineering and Biotechnology, 10, 1024143.
Castro, K. C. D., Costa, J. M., & Campos, M. G. N. (2022). Drug-loaded polymeric nanoparticles: A review. International Journal of Polymeric Materials and Polymeric Biomaterials, 71(1), 1-13.
Imazato, S., Kitagawa, H., Tsuboi, R., Kitagawa, R., Thongthai, P., & Sasaki, J. I. (2017). Non-biodegradable polymer particles for drug delivery: A new technology for “bio-active” restorative materials. Dental Materials Journal, 36(5), 524-532.
Mukherjee, C., Varghese, D., Krishna, J. S., Boominathan, T., Rakeshkumar, R., Dineshkumar, S., … & Sivaramakrishna, A. (2023). Recent advances in biodegradable polymers–properties, applications and future prospects. European Polymer Journal, 192, 112068.
Rasul, R. M., Muniandy, M. T., Zakaria, Z., Shah, K., Chee, C. F., Dabbagh, A., … & Wong, T. W. (2020). A review on chitosan and its development as pulmonary particulate anti-infective and anti-cancer drug carriers. Carbohydrate Polymers, 250, 116800.
Krishnan, K. M. Introduction to Materials Characterization, Analysis, and Metrology. In Principles of Materials Characterization and Metrology (pp. 1-67). Oxford University Press.
Baer, D. R., Engelhard, M. H., Johnson, G. E., Laskin, J., Lai, J., Mueller, K., … & Moon, D. (2013). Surface characterization of nanomaterials and nanoparticles: Important needs and challenging opportunities. Journal of Vacuum Science and Technology A: Vacuum, Surfaces and Films, 31(5), 050820.
Vos, M. (2023). Electron scattering at high momentum transfer. Journal of Electron Spectroscopy and Related Phenomena, 267, 147382.
Skowyra, M. M., Ankjærgaard, C., Yu, L., Lindvold, L. R., Skov, A. L., & Miller, A. (2022). Characterization of a radiofluorogenic polymer for low-energy electron beam penetration depth visualization. Polymers, 14(5), 1015.
Mourdikoudis, S., Pallares, R. M., & Thanh, N. T. (2018). Characterization techniques for nanoparticles: comparison and complementarity upon studying nanoparticle properties. Nanoscale, 10(27), 12871-12934.
Larivière, D., Tolmachev, S. Y., Kochermin, V., & Johnson, S. (2013). Uranium bone content as an indicator of chronic environmental exposure from drinking water. Journal of Environmental Radioactivity, 121, 98-103.
Lin, P. C., Lin, S., Wang, P. C., & Sridhar, R. (2014). Techniques for physicochemical characterization of nanomaterials. Biotechnology Advances, 32(4), 711-726.
Jagadeesh, P., Rangappa, S. M., & Siengchin, S. (2024). Advanced characterization techniques for nanostructured materials in biomedical applications. Advanced Industrial and Engineering Polymer Research, 7(1), 122-143.
Prasad, R. D., Sonawane, K. D., Prasad, R. S., Prasad, S. R., Prasad, N., Shrivastav, R. K., … & Guo, Z. (2024). A review on livestock viral disease and their management. ES General, 5, 1227.
Redasani, V. K., Patel, P. R., Marathe, D. Y., Chaudhari, S. R., Shirkhedkar, A. A., & Surana, S. J. (2018). A review on derivative uv-spectrophotometry analysis of drugs in pharmaceutical formulations and biological samples review. Journal of the Chilean Chemical Society, 63(3), 4126-4134.
Swinehart, D. F. (1962). The beer-lambert law. Journal of Chemical Education, 39(7), 333.
Krajczewski, J., Kołątaj, K., & Kudelski, A. (2017). Plasmonic nanoparticles in chemical analysis. RSC Advances, 7(28), 17559-17576.
Zia, K., Siddiqui, T., Ali, S., Farooq, I., Zafar, M. S., & Khurshid, Z. (2019). Nuclear magnetic resonance spectroscopy for medical and dental applications: a comprehensive review. European Journal of Dentistry, 13(01), 124-128.
Prasad, R. D., Prasad, N. R., Prasad, N., Prasad, R. S., Prasad, S. R., Shrivastav, O. P., … & Saxena, P. (2024). A review on phenomenon of adsorption of inorganic materials: applications in veterinary pharmacology and material sciences. Advances in Analytic Science, 5(2), 3000.
Prasad, R. D., Prasad, N. R., Prasad, R. S., Shrivastav, O. P., Prasad, S. R., Banga, S., … & Prasad, R. R. (2024). A review on nanotechnological aspects in medicinal textile. Advances in Analytical Sciences, 5, 2694.
Navathe, G. J., Prasad, S. R., Mane, A. M., Barge, S. H., Dongale, T. D., Shaikh, V., … & Prasad, N. R. (2022). A critical review on design and development of new generation energy storage devices. ES Energy & Environment, 17(2), 11-32.