The combination of polyurethane (PU) and phosphates is recognized for forming effective flame retardant (FR) intumescent systems. However, the flame retardant properties can be compromised due to the water solubility of phosphates, making the intumescent coating non-permanent. To address this issue, microencapsulation techniques were employed, encapsulating di-ammonium hydrogen phosphate (DAHP) within a polyurethane shell. The chemical and physical structures of the DAHP microcapsules were characterized to assess their stability and performance. Cotton fabrics were coated with polyurethane formulations containing either neat DAHP or encapsulated DAHP. Results demonstrated that microencapsulation of DAHP within a polyurethane shell significantly enhanced the flame retardant performance of the coated cotton.
Polyurethane (PU) coatings are widely used in the textile industry due to their ability to enhance properties such as abrasion resistance, water repellence, and the imitation of leather textures. PU-coated fabrics, particularly cotton and cotton-polyester blends, are employed across various applications, including automotive interiors, waterproof garments, and furniture upholstery. However, despite these advantages, PU coatings exhibit poor flame behavior, which poses a challenge given the increasingly stringent fire safety regulations for materials used in public domains.
To improve the fire resistance of PU-coated fabrics, the integration of flame retardants (FRs) has proven effective. Among these, ammonium phosphates have been identified as promising additives for creating an intumescent flame retardant system when combined with PU. Intumescent systems work by forming an insulating, charred barrier on the material’s surface when exposed to heat, thereby protecting the underlying fabric from flames and delaying combustion. This expanded char layer limits the transfer of heat and fuel to the gas phase, offering a significant reduction in fire risk.
Intumescent formulations typically contain three key components: an acid source (e.g., phosphates), a carbon source (e.g., polyols), and a blowing agent (e.g., melamine). Ammonium phosphates serve as an effective acid source, facilitating the dehydration of carbon, while the release of ammonia gas from their decomposition contributes to the formation of the insulating char layer. However, a major limitation of ammonium phosphates is their water solubility and weak affinity with polymer matrices, leading to issues such as migration and reduced long-term effectiveness of the flame retardant system.
To overcome these limitations, microencapsulation of ammonium phosphates has been proposed. Microencapsulation involves surrounding the flame retardant agent with a protective polymer shell, which isolates it from environmental factors such as water and enhances its stability. Polyurethane has been identified as a suitable material for the encapsulating shell, given its compatibility with PU-coated textiles and other polymer matrices. Moreover, the combination of encapsulated ammonium phosphates with a PU shell can serve as an effective intumescent system, offering durable flame resistance.
This study focuses on the development and evaluation of microencapsulated di-ammonium hydrogen phosphate (DAHP) for use in PU-coated cotton fabrics. The synthesis and characterization of DAHP microcapsules with a PU shell are presented, followed by an assessment of the flame-retardant properties of these coated fabrics using a cone calorimeter. The cone calorimeter, a reliable model for fire testing, measures the rate of heat release, which is critical for understanding the material’s fire behavior. The findings of this study demonstrate the potential of microencapsulation to enhance the flame resistance of PU-coated textiles and meet the growing demands for fire-safe materials in various industries.
The raw materials used in this study were employed without further purification (Table 1). Di-ammonium hydrogen phosphate (DAHP) [(NH\(_4\))\(_2\)HPO\(_4\), purity 99%, Riedel-de Haën AG], diphenyl methylene diisocyanate (MDI) (Suprasec 2030, Hüntsman ICI; a blend of MDI isomers, primarily 4,4´-diphenyl methylene diisocyanate), polyethylene glycol 400 g/mol (PEG 400, Prolabo), and the catalyst dibutyl tin dilaureate (DBTDL) (Aldrich) were used. Toluene (Verbièse) served as the organic phase, while a triblock copolymer ABA, where A is an oleate and B is polyethylene oxide (400 g/mol), was used as the surfactant. A pure cotton fabric (146 g/m²) was selected as the substrate for coating.
The polyurethane (PU) coating was prepared using a mixture of 100 parts polyol (Allrim polyol AW) and 140 parts MDI prepolymer (Allrim isocyanate BY). Neat DAHP or encapsulated DAHP was incorporated into the PU paste at concentrations of 15–30% by weight.
The microencapsulation of DAHP followed the standard procedure for encapsulating water-soluble substances with a PU shell. Three separate solutions were prepared:
Solution I: 2.5 g of surfactant was dissolved in 225 mL of toluene.
Solution II: 6.67 g of DAHP and 3.15 g of PEG 400 were dissolved in 45 mL of water.
Solution III: 1.77 g of MDI and 0.12 g of DBTDL were dissolved in 37.5 mL of solution I.
Solution II was added to a 500 mL reactor containing the remainder of solution I, under constant stirring at 700 rpm for 5 minutes. Solution III was then introduced, and stirring was maintained for an additional 5 minutes. The stirring speed was subsequently reduced to 300 rpm, and the reaction proceeded for 4 hours at 63°C. Following the reaction, the microcapsules were recovered by filtration, rinsed with toluene and water, and dried at 40°C for 24 hours. This process was repeated several times, yielding consistent microcapsule characteristics, confirming the reliability and reproducibility of the synthesis.
| Material | Supplier |
| Di-ammonium hydrogen phosphate (DAHP) | Riedel-de Haën AG |
| Diphenyl methylene diisocyanate (MDI) | Hüntsman ICI |
| Polyethylene glycol 400 g/mol (PEG 400) | Prolabo |
| Dibutyl tin dilaureate (DBTDL) | Aldrich |
| Toluene | Verbièse |
| Triblock copolymer ABA surfactant | Custom |
| Polyol (Allrim polyol AW) | Allrim |
| MDI prepolymer (Allrim isocyanate BY) | Allrim |
The microcapsules were observed using an Axioskop ZEISS optical microscope equipped with an IVC 800 12S camera. Their size distribution was characterized using an Accusizer 770 Particle Sizing System, which detects particle diameters based on laser diode illumination. The particles were dispersed in an aqueous solution containing 1 wt% sodium dodecyl sulfate (SDS), and particle size distribution was measured by comparing pulse heights with a standard calibration curve.
Fourier-transform infrared (FTIR) spectra were recorded using a Nicolet Nexus spectrometer in transmission mode. The samples were ground and mixed with KBr to form pellets. Spectra were obtained from 32 scans with a resolution of 4 cm\(^{-1}\).
Solid-state \(^{13}\)C and \(^{31}\)P NMR measurements were performed using a Bruker ASX 100 spectrometer. For \(^{13}\)C NMR, spectra were obtained with cross-polarization (CP) and magic angle spinning (MAS) at 12 kHz. \(^{31}\)P NMR was performed with a repetition time of 450 s and 85% H\(_3\)PO\(_4\) as a reference.
The cotton fabrics were coated using a K Control Coater (Erichsen), employing a technique similar to coating with a scraper. The coating paste was spread with a threaded rod, achieving a theoretical thickness of 36 \(\mu\)m. The coated fabrics were then dried in an oven at 80°C for 4 hours. The weight of the deposited coating was approximately 210 g/m². Various formulations of PU coatings with neat and encapsulated DAHP were prepared for comparison.
Fire performance of the samples was evaluated using a Stanton Redcroft Cone Calorimeter in accordance with ASTM E 1354, a standard test method for measuring heat and visible smoke release rates. The samples (9 \(\times\) 9 cm²) were exposed to a heat flux of 35 kW/m², a typical flux for mild fire scenarios. The tests were repeated three times, and conventional data such as the rate of heat release (RHR), volume of smoke production (VSP), weight loss, and CO and CO\(_2\) emissions were measured. The results are presented as averages, with reproducibility within ±10% for RHR and VSP, and ±15% for weight loss and gas emissions.
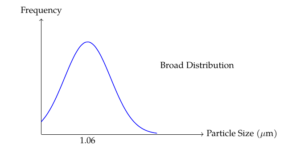
The DAHP microcapsules synthesized for this study were found to be almost spherical in shape (Figure 1). Some microcapsules were slightly stuck together, but their overall morphology remained consistent. The microcapsule size ranged between 0.5 and 5 \(\mu\)m, with a number-average diameter of approximately 1.06 \(\mu\)m. The particle size distribution was found to be slightly broad (Figure 2), which is attributed to the blade geometry used during the microcapsule synthesis process. It is expected that altering the propeller geometry or increasing the stirring speed during the emulsion stage may result in a narrower particle size distribution. Importantly, the microcapsules are small enough (less than 5 \(\mu\)m) to be used in textile coatings without being damaged during the coating process by the scraper.
The FTIR spectrum of DAHP microcapsules (Figure 3) shows characteristic bands for MDI and PEG 400 monomers, including the CH\(_2\) group at 2919 cm\(^{-1}\) and aromatic groups at 3033, 1594, 1413, and 817 cm\(^{-1}\), which correspond to MDI. The ether group of PEG 400 is observed at 1112 cm\(^{-1}\). Notably, the absence of the isocyanate peak at 2268 cm\(^{-1}\) indicates complete reaction of MDI with PEG 400 and water, leading to the formation of urethane and urea groups. These groups are observed through the overlapped peaks at 1705 cm\(^{-1}\) (C=O for urethane) and 1660 cm\(^{-1}\) (C=O for urea), as well as N–H stretching at 3340, 1538, and 1235 cm\(^{-1}\).
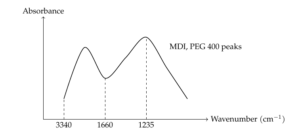
Solid-state \(^{31}\)P and \(^{13}\)C NMR further confirmed the successful encapsulation of DAHP. The \(^{31}\)P NMR spectrum (Figure 3) shows a phosphate peak at 0 ppm, indicating that DAHP is encapsulated within the microcapsules. Interestingly, the phosphate band is narrower in the microcapsules than in neat DAHP, suggesting a higher symmetry of the tetrahedral PO\(_4\) group within the microcapsule structure. The \(^{13}\)C NMR spectrum (Figure 3) shows chemical shifts for MDI, with peaks at 123, 130, and 137 ppm corresponding to aromatic carbons, and 41 ppm for CH\(_2\). Peaks at 71 and 156 ppm correspond to the OCH\(_2\) group of PEG 400 and the carbonyl group of urethane, respectively, indicating successful polycondensation between PEG 400 and MDI.
The rate of heat release (RHR) curves (Figure 4) demonstrate that PU coatings loaded with DAHP microcapsules have significantly improved fire resistance compared to virgin PU. The RHR peak of virgin PU-coated cotton was observed at 340 kW/m\(^2\), while it decreased by 12% with 15 wt% DAHP microcapsules and by 25% with 30 wt% DAHP microcapsules. Despite the lower DAHP content in the PU coatings with microcapsules compared to neat DAHP, the performance in terms of fire retardancy was similar. It is estimated that 15 wt% and 30 wt% of DAHP microcapsules correspond to approximately 8 wt% and 18 wt% of neat DAHP in terms of fire retardant performance.
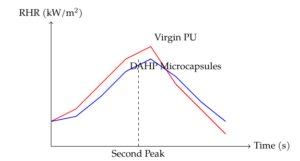
Interestingly, a second RHR peak was observed for virgin PU, 15% DAHP microcapsules, and 30% DAHP microcapsules, indicating surface crack formation. For virgin PU, this second peak led to complete sample decomposition, while for 15% DAHP microcapsules, the peak was significantly flattened and delayed. The 30% DAHP microcapsule formulation exhibited a third peak, which corresponded to the cracking of the multicellular surface and the formation of foamed intumescent char. In contrast, formulations with neat DAHP did not show a second peak, suggesting better resistance to heat and flame stresses.
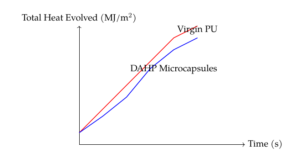
The total heat evolved (THE) during combustion for all coating formulations is presented in Figure 5. The 30% DAHP formulation exhibited a slower flame spread compared to other formulations, as indicated by the shallower slope of the THE curve. The flame spread for microcapsule formulations was intermediate between virgin PU and neat DAHP formulations. While neat DAHP formulations significantly delayed the combustion compared to virgin PU, microcapsule formulations exhibited delayed combustion up to 150 seconds. However, except for the 30 wt% DAHP formulation, the THE values for fire retardant (FR) coatings were higher than those of virgin PU, indicating that the formulations primarily act as flame retardants rather than enhancing fire resistance.
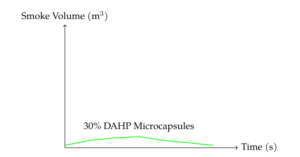
The volume of smoke production (VSP) during combustion is shown in Figure 6. The formulation containing 30 wt% DAHP microcapsules showed a peak smoke volume of 0.006 m\(^3\) at 12 seconds, slightly higher than other formulations, but overall, the VSP curves were similar across all samples.
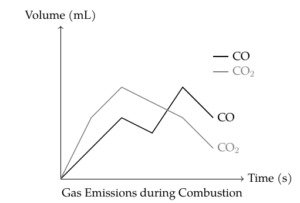
Figures 7 present the amount of CO and CO\(_2\) released during combustion. The formulations with 20% and 30% neat DAHP exhibited lower CO\(_2\) emissions and higher CO emissions, indicating incomplete combustion and better development of the intumescent system. However, the differences in CO and CO\(_2\) emissions between the various coatings were not very significant, suggesting that both neat and microencapsulated DAHP formulations provide similar fire retardancy effects.
The study successfully demonstrated the synthesis and characterization of microencapsulated di-ammonium hydrogen phosphate (DAHP) using a polyurethane (PU) matrix, showcasing the potential of these microcapsules for flame-retardant applications in textile coatings. The nearly spherical morphology of the microcapsules, with a number-average diameter of approximately 1.06 \(\mu\)m, confirmed their suitability for integration into the PU coating process without risk of damage.
Characterization techniques, including FTIR and NMR spectroscopy, confirmed the successful encapsulation of DAHP within the PU shell. The absence of isocyanate peaks in the FTIR spectrum indicated complete reaction with the polyol, resulting in the formation of stable urethane and urea groups.
Fire testing revealed that incorporating DAHP microcapsules into the PU coating significantly reduced the rate of heat release (RHR), with a decrease of up to 25% at higher loading levels. This indicates enhanced flame-retardant properties compared to virgin PU coatings. Additionally, the formulations containing microencapsulated DAHP exhibited improved thermal stability and reduced flame spread, particularly at higher concentrations.
While the formulations with microencapsulated DAHP demonstrated flame-retardant effects, the total heat evolved (THE) and gas emissions, particularly \(CO\) and \(CO_2\), indicated that further optimization is needed to enhance fire resistance. The production of smoke varied with the formulation, suggesting that the encapsulation method influences combustion characteristics.
Overall, the findings suggest that DAHP microcapsules within PU coatings can be an effective approach for developing flame-retardant textiles, although further investigations are warranted to refine their performance and understand the mechanisms underlying their fire-retardant effects.
Giraud, Stephane, et al. “Microencapsulation of phosphate: application to flame retarded coated cotton.” Polymer Degradation and Stability 77.2 (2002): 285-297.
Giraud, Stéphane, et al. “Flame retarded polyurea with microencapsulated ammonium phosphate for textile coating.” Polymer degradation and stability 88.1 (2005): 106-113.
Liu, Shang-Hao, et al. “Preparation and flame retardance of polyurethane composites containing microencapsulated melamine polyphosphate.” Polymers 9.9 (2017): 407.
Shen, Ming-Yuan, et al. “Preparation, characterization of microencapsulated ammonium polyphosphate and its flame retardancy in polyurethane composites.” Materials Chemistry and Physics 173 (2016): 205-212.
Liu, Shang-Hao, et al. “Preparation, characterization and its flame retardance performance of microencapsulated ammonium polyphosphate/bridged polysesquisiloxane polyurethane composites.” Journal of Polymer Research 23 (2016): 1-9.
Liu, Hong, Bin Zhang, and Jian Han. “Improving the flame retardancy and smoke suppression properties of polyurethane foams with SiO2 microcapsule and its flame-retardant mechanism.” Polymer-Plastics Technology and Engineering 57.11 (2018): 1139-1149.
Xu, Ying-Jun, et al. “An overview of alginates as flame-retardant materials: Pyrolysis behaviors, flame retardancy, and applications.” Carbohydrate polymers 260 (2021): 117827.
Bomba, Poliestrno IN, and NO BLAGO. “Application of flame retardant microcapsules to polyester and cotton fabrics.” Materiali in tehnologije 48.1 (2014): 105-111.
Kozlowski, R., et al. “Intumescent flame-retardant treatments for flexible barriers.” Multifunctional Barriers for Flexible Structure: Textile, Leather and Paper (2007): 39-61.
Majlingováa, Andrea, and Danica Kačíkováa–Qiang Xub–Cong Jinb. “Current trends in flame-retardant treatment of selected polymers–a review.” Earth 2.0 (2018).
Zhang, Yan-Kui, et al. “Influence of microencapsulation on combustion behavior and thermal degradation of intumescent flame-retarded epoxy composite.” Polymer-Plastics Technology and Engineering 51.10 (2012): 1054-1061.