Wireless capsule endoscopy (WCE) continues to transform gastrointestinal (GI) diagnostics by offering a non-invasive, patient-friendly alternative for examining the GI tract. Recent advancements have significantly improved its efficiency and image quality, providing enhanced diagnostic capabilities. The next phase in WCE development envisions capsules not only diagnosing but also performing therapeutic interventions, such as drug delivery, tissue biopsy, and polyp removal. However, realizing this vision presents several technical challenges, particularly in achieving precise control of the capsule’s movement (actuation) and accurate localization within the complex GI environment. This paper reviews the current progress in overcoming these challenges, highlights recent technological advancements, and explores future solutions aimed at making WCE an integrated diagnostic and therapeutic tool.
Endoscopy, a vital diagnostic tool in modern medicine, refers to the practice of visualizing the interior of the body to examine organ function or identify abnormalities. The concept of endoscopy dates back to the early 19th century when Philipp Bozzini developed the first rigid endoscope, the Lichtleiter (light conductor), in 1806 for inspecting the urinary tract and bladder. Although revolutionary for its time, it was not until the development of flexible fiber-optic technology in the 1950s that endoscopy made significant strides in clinical diagnostics. This “second generation” of endoscopes allowed for more versatile, flexible procedures and enabled physicians to observe internal structures with greater accuracy and less discomfort to the patient.
The evolution continued into the 1980s with the introduction of electronic endoscopes, which integrated a charge-coupled device (CCD) camera. These devices converted images into electrical signals, allowing them to be displayed on a monitor, thus permitting real-time viewing by medical professionals. This advancement also allowed for greater collaboration, as multiple practitioners could observe and analyze the same procedure. As a result, gastrointestinal (GI) endoscopy became an indispensable tool for diagnosing and treating diseases affecting the digestive system, such as ulcers, cancers, and polyps. Modern GI endoscopes are equipped with advanced features, enabling doctors to inspect the esophagus, stomach, duodenum, and colon with precision. However, despite the tremendous progress, traditional endoscopy is often invasive, requiring the insertion of a scope through the mouth or anus, leading to discomfort and complications such as bleeding or perforation.
A transformative innovation emerged in 2000 with the advent of wireless capsule endoscopy (WCE), introduced by the company Given Imaging. This technology, detailed in a seminal article by [1], featured a pill-sized capsule containing a tiny camera, light-emitting diodes (LEDs), and a wireless transmitter. The capsule, once swallowed, traverses the GI tract, capturing thousands of images, particularly of the small intestine—a region that traditional endoscopes often struggle to access. These images are transmitted wirelessly to sensors placed on the patient’s abdomen, which then send the data to a recording device for further analysis by the physician. The development of this wireless, non-invasive technology revolutionized the field of gastrointestinal diagnostics [2]. It addressed some of the key limitations of conventional endoscopy, such as patient discomfort, invasiveness, and the inability to access certain parts of the GI tract without surgery [3].
The wireless capsule endoscope (WCE) has since been commercialized under various names, such as PillCam, and has been used successfully in diagnosing conditions like Crohn’s disease, obscure GI bleeding, and tumors. The PillCam consists of a capsule measuring approximately 11 mm x 26 mm, equipped with light sources, a camera, a wireless transmission module, and a battery capable of operating for up to eight hours. Patients simply swallow the capsule with water, and it naturally moves through the digestive system, taking images that are used for diagnostics [4]. The capsule is excreted within a few days, eliminating the need for retrieval.
Despite its revolutionary potential, WCE is currently limited to diagnostic functions, and the capsule cannot be actively controlled once swallowed. It moves passively through the digestive system, relying on peristalsis for propulsion [5]. This presents significant limitations, particularly in situations where a more detailed examination is required, or in cases where a capsule may need to be repositioned or directed to a specific area of interest. In such cases, the capsule may pass too quickly through a region of the GI tract or may not capture sufficient images of a suspicious area. In addition, the capsule cannot perform therapeutic interventions, such as tissue biopsy or the removal of polyps [6].
To overcome these limitations, researchers are exploring the development of active capsule endoscopes—devices that can be externally controlled to adjust their speed, direction, and orientation, and eventually even perform therapeutic tasks [7]. Achieving this goal requires addressing two major challenges: actuation and localization. Actuation refers to the ability to control the movement of the capsule within the GI tract, potentially enabling it to stop, change direction, or remain in place for prolonged observation or intervention. Localization, on the other hand, is the ability to determine the exact position of the capsule within the body at any given time, providing precise information about its location relative to anatomical landmarks or abnormalities [8].
Current research efforts are focused on overcoming the technical and physiological challenges of implementing these features in a small, self-contained, swallowable capsule. Various methods of actuation have been proposed, ranging from magnetic fields and bio-inspired mechanisms to electrical stimulation and micro-motors [9]. Likewise, localization technologies are being developed to track the capsule’s position in real time with millimeter-level accuracy, essential for both diagnosis and intervention. As wireless capsule endoscopy continues to evolve, it is expected that future generations of these devices will not only diagnose but also treat gastrointestinal diseases, making them indispensable tools in modern medicine.
This paper explores the current state of active capsule endoscopy, focusing on the technological advances in actuation and localization. It also highlights the remaining challenges and proposes potential solutions to address these hurdles, aiming to transition capsule endoscopes from passive diagnostic tools to active therapeutic devices.
The introduction of capsule endoscopy (CE) marked a paradigm shift in the field of gastrointestinal (GI) diagnostics, offering a non-invasive and patient-friendly alternative to conventional endoscopy. Capsule endoscopy emerged as a result of technological advancements in miniaturization, wireless communication, and medical imaging, enabling a device small enough to be swallowed by the patient yet sophisticated enough to capture thousands of images from within the GI tract. The development of this technology opened up new possibilities, particularly for diagnosing conditions in the small intestine, which was previously challenging due to the limitations of traditional endoscopy techniques.
Capsule endoscopy was first introduced commercially by Given Imaging in 2000, under the brand name PillCam. The original PillCam, also known as M2A (mouth-to-anus), contained a camera, light sources, a battery, and a wireless transmitter, all encased within a small capsule measuring approximately 11 mm in diameter and 26 mm in length. Once swallowed, the capsule would travel naturally through the gastrointestinal tract, propelled by peristalsis—the rhythmic contraction of muscles that moves food through the digestive system. The capsule captures images of the inner lining of the GI tract at regular intervals and transmits these images wirelessly to sensors placed on the patient’s abdomen. The data is then sent to a portable recording device worn by the patient, which can later be analyzed by medical professionals [10].
The key innovation of capsule endoscopy lies in its ability to provide visual access to the small intestine, a part of the GI tract that is difficult to examine using traditional endoscopy methods. Conventional endoscopes, such as gastroscopes and colonoscopes, are limited by their length and flexibility, which restricts their ability to reach deep into the small intestine. The small intestine, which measures approximately 6 meters in length, is crucial in diagnosing conditions like Crohn’s disease, obscure gastrointestinal bleeding, and small bowel tumors. Capsule endoscopy, by contrast, allows for a complete visualization of this area, offering a new diagnostic tool that has become indispensable for gastroenterologists.
Since its initial introduction, capsule endoscopy technology has evolved significantly, with various manufacturers developing their own versions of the device. The PillCam series, manufactured by Given Imaging, remains one of the most widely used capsule endoscopy systems, but other companies such as Olympus and Jinshan have also developed their own devices, such as the EndoCapsule and OMOM, respectively [3].
Each of these systems shares common characteristics, though they differ in certain technical specifications. Below is a detailed comparison of the features of major commercially available capsule endoscopy systems.
PillCam SB/SB2 (Given Imaging): The original PillCam was designed specifically for the small intestine. The latest models, such as the SB2, feature a camera capable of capturing images at two frames per second, with a field of view between 140 and 156 degrees. The capsule operates for up to 8 hours, providing ample time for a complete examination of the small bowel. It transmits images wirelessly to an external receiver via radiofrequency (RF) signals, allowing doctors to retrieve detailed images of the small intestine for later analysis [2].
PillCam ESO (Given Imaging): The PillCam ESO is a modified version of the original PillCam designed specifically for esophageal examinations. It includes two cameras (one on each end of the capsule) that allow for a faster frame rate of up to 14 frames per second. This higher frame rate is necessary for capturing images of the esophagus, which has a shorter transit time compared to the small intestine [1].
PillCam Colon (Given Imaging): The PillCam Colon is designed to examine the large intestine, particularly in patients who cannot undergo traditional colonoscopy. It features two cameras, similar to the PillCam ESO, and incorporates advanced features such as automatic power management. The capsule deactivates after three minutes of operation, conserving battery life, and reactivates after 105 minutes when it is expected to have reached the large intestine [5].
EndoCapsule (Olympus): The EndoCapsule from Olympus is similar to the PillCam SB but incorporates real-time image display capabilities, allowing physicians to monitor the capsule’s progress through the GI tract during the procedure. This real-time feature provides additional diagnostic flexibility [4].
OMOM Capsule (Jinshan): The OMOM capsule endoscope is another popular system, offering a compact design and similar capabilities to the PillCam. It uses a complementary metal-oxide-semiconductor (CMOS) sensor, has a field of view of 140 degrees, and can operate for 6 to 8 hours [7].
A comparison of the technical specifications of these systems highlights the common design constraints in capsule endoscopy: capsule size, battery life, image resolution, and transmission capabilities. All commercially available capsule endoscopes must strike a balance between miniaturization and performance. The small size of the capsule is critical to ensure that it can be safely swallowed by the patient, yet it must also contain all necessary components, including the camera, light sources, battery, and wireless transmitter. Current capsule endoscopes are powered by small button batteries, which typically provide up to 8 hours of operation. However, battery life is a limiting factor, particularly for capsules designed to explore the entire gastrointestinal tract, including the colon.
Capsule endoscopy is most commonly used to diagnose conditions affecting the small intestine. Obscure gastrointestinal bleeding, which cannot be detected using traditional endoscopy methods, is one of the most frequent indications for capsule endoscopy. The capsule can capture images of the entire small bowel, helping doctors locate sources of bleeding, such as ulcers, tumors, or vascular malformations. This capability is particularly important for patients who have experienced unexplained GI bleeding and have had inconclusive results from other diagnostic procedures.
Another major application of capsule endoscopy is in the diagnosis and management of Crohn’s disease, a chronic inflammatory condition that primarily affects the small intestine. In Crohn’s disease, inflammation can occur anywhere in the GI tract, but it is most often found in the ileum, the final part of the small intestine. Capsule endoscopy allows for a non-invasive and detailed examination of the small bowel, aiding in the diagnosis of Crohn’s disease and monitoring disease progression over time.
Capsule endoscopy is also used to detect small bowel tumors, which are relatively rare but difficult to diagnose using conventional methods. The small intestine is a common site for tumors in patients with familial adenomatous polyposis (FAP) and Peutz-Jeghers syndrome, two genetic conditions that predispose individuals to develop multiple polyps and tumors in the GI tract. Capsule endoscopy provides a safe and effective means of screening these patients for early-stage tumors, potentially leading to earlier interventions and better outcomes.
While capsule endoscopy is predominantly used for small bowel examinations, newer versions, such as the PillCam Colon, have been developed to explore other regions of the GI tract, including the esophagus and colon. The PillCam Colon, for instance, offers a less invasive alternative to traditional colonoscopy for patients who may not be able to tolerate the procedure due to age, medical conditions, or previous complications. This version of the capsule endoscope is designed to navigate the twists and turns of the large intestine, capturing high-resolution images that can be used to identify polyps, tumors, and other abnormalities.
Despite its many advantages, capsule endoscopy has several limitations that researchers are actively seeking to address. One of the primary limitations is that current capsule endoscopes are passive devices, meaning they cannot be controlled once swallowed. They rely entirely on natural peristalsis to move through the GI tract, which can result in variable transit times and inconsistent image capture [11]. For example, if the capsule passes too quickly through a particular region, it may not capture sufficient images to detect a pathology. Conversely, if the capsule becomes lodged or slows down in a certain area, it may cause unnecessary delays in completing the examination.
Another limitation is that capsule endoscopes cannot perform therapeutic procedures, such as taking biopsies, removing polyps, or stopping bleeding. If a suspicious area is identified during the examination, the patient must undergo a follow-up procedure using traditional endoscopy or surgery to obtain a tissue sample or perform treatment.
The current generation of capsule endoscopes (CE) is primarily passive, relying on the natural movement of the gastrointestinal (GI) tract, called peristalsis, to travel through the body. While this passive movement allows for non-invasive and relatively straightforward diagnostics, it also comes with several limitations, such as inconsistent transit times and the inability to stop or steer the capsule for more detailed examinations. To overcome these challenges, researchers are developing active capsule endoscopes, which can be controlled to change their position, speed, and orientation within the GI tract. The primary focus of these advancements is on actuation mechanisms, which enable controlled movement and navigation through the often complex and unpredictable environment of the GI system [12].
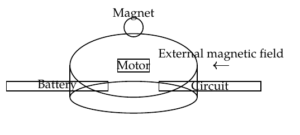
Internal actuation mechanisms rely on technologies housed entirely within the capsule to generate motion. These mechanisms mimic biological systems or use mechanical components like motors, actuators, or shape memory alloys (SMAs) to control the movement of the capsule.
| Aspect | Current Technology | Performance (2024) | Potential Target |
| Capsule Size | 11 mm x 26 mm | 11 mm x 20 mm (smaller models available) | 8 mm x 20 mm |
| Image Resolution | 640 x 480 pixels (VGA) | 1920 x 1080 pixels (Full HD) | 4K (3840 x 2160 pixels) |
| Battery Life | 8 hours | 12 hours | 24 hours (with energy harvesting) |
| Frame Rate | 2-4 fps | 18 fps (PillCam ESO) | 24 fps |
| Localization Accuracy | 3 cm (RF-based) | 3 mm (magnetic localization) | Sub-millimeter (with hybrid localization) |
| Actuation Speed | Passive movement (via peristalsis) | 4.5 mm/s (internal SMA or micromotor-based) | 10 mm/s (magnetic or hybrid actuation) |
| Therapeutic Tools | None | Basic biopsy and drug delivery prototypes | Fully integrated therapeutic modules (biopsy, drug delivery, polyp removal) |
| Data Transmission Rate | 500 Kbps | 1 Mbps | 5 Mbps (real-time imaging and control) |
| Field of View | 140 degrees | 170 degrees (dual cameras) | 180 degrees (with panoramic view) |
| Operating Time | 8 hours | 10-12 hours | 24 hours (with external power support) |
One of the most promising internal actuation methods involves shape memory alloys (SMA), which are materials that can change shape when exposed to different temperatures. In capsule endoscopy, SMAs can be used to mimic the movement of legs or paddles, propelling the capsule through the GI tract. These bio-inspired designs often include small mechanical legs or micro-hooks that push against the walls of the intestine to generate movement. The legs, typically made from SMAs, can contract and expand in response to temperature changes caused by electrical currents. This method allows for slow, controlled movement through the intestine, and it has been shown to work effectively in laboratory environments. However, the application of SMAs in a clinical setting poses challenges, such as the difficulty of managing heat dissipation within the body and ensuring that the actuation system does not cause harm to surrounding tissues.
Micromotors are another internal actuation mechanism being explored for capsule endoscopy. These miniature motors can generate rotational or linear motion within the capsule, allowing for more controlled movement. For example, micromotors can be used to drive paddles or wheels that propel the capsule forward. One such design involves a stepper micromotor attached to a threaded rod inside the capsule. As the motor rotates, the rod converts rotational motion into linear motion, pushing the capsule forward. In laboratory tests, these micromotor-driven capsules have achieved movement speeds of several millimeters per second, making them promising candidates for controlled navigation in the GI tract.
Bio-inspired designs are also gaining traction as potential internal actuation solutions. These designs often mimic the natural movement mechanisms found in animals or other biological systems. For example, some capsules are equipped with small legs or cilia-like structures that allow them to crawl along the intestinal walls. Other designs draw inspiration from earthworms or caterpillars, using peristaltic motion to move the capsule forward in a smooth, continuous manner.
| Aspect | Current Technology | Limitations | Potential Advancements |
| Capsule Size and Design | Small capsule (11 mm x 26 mm), self-contained | Limited space for adding additional components (actuators, therapeutic tools) | More compact designs, integration of multiple robotic capsules, improved internal components |
| Movement Mechanism | Passive movement via peristalsis | Inconsistent transit times, cannot stop or control direction | Active actuation mechanisms (internal micromotors, SMA legs, external magnetic or electromagnetic control) |
| Internal Actuation | SMA-based bio-inspired legs, micromotors | Heat dissipation, slow response, power limitations | Improved heat management, faster response micromotors, bio-inspired systems mimicking biological movement |
| External Actuation | Magnetic actuation (external magnetic fields control the capsule) | Requires close proximity for control, weakened force with distance | Enhanced magnetic control systems, combination of internal and external actuation |
| Localization | RF-based signal triangulation, magnetic localization | Low accuracy ( centimeter level), interference from body tissues and external magnetic fields | Millimeter-level accuracy with advanced magnetic localization, AI-driven hybrid localization (magnetic, RF, ultrasound) |
| Power Supply | Small button batteries (up to 8 hours operation) | Limited operational time, insufficient for both imaging and active movement | Wireless power transmission, high-density batteries, biologically generated energy |
| Imaging and Diagnostics | CMOS sensors, light sources for imaging | Cannot perform therapeutic interventions (e.g., biopsies, drug delivery) | Real-time localization enabling precise therapeutic actions (biopsies, drug delivery systems, polyp removal) |
| Therapeutic Potential | None (diagnostic only) | Cannot perform treatments, remove polyps, or take biopsies | Integration of therapeutic tools for localized treatments, biopsies, drug delivery systems |
| Challenges | Power management, miniaturization, actuation/localization conflicts | Difficulty in precise control, short power lifespan | Wireless power, smaller yet more powerful batteries, integrated actuation and localization without interference |
In contrast to internal systems, external actuation mechanisms rely on forces generated outside the body to control the movement of the capsule. These methods are particularly attractive because they do not require the capsule to carry a power source or complex mechanical components, thereby reducing the size and complexity of the device.
Magnetic actuation is one of the most widely researched external methods for controlling capsule endoscopes. In this approach, the capsule is equipped with a small magnet, and an external magnetic field is applied to manipulate the capsule’s movement. By adjusting the strength and orientation of the external magnetic field, the capsule can be guided through the GI tract, changing its speed and direction as needed. Magnetic actuation offers significant advantages in terms of power consumption, as the capsule does not require an internal power source for movement [13]. However, the effectiveness of magnetic actuation is influenced by several factors, including the strength of the magnetic field, the distance between the magnet and the external controller, and the resistance encountered within the intestine. Achieving precise control over the capsule’s movement in a clinical setting remains a challenge, particularly when navigating complex or collapsed sections of the GI tract.
Another external actuation technique involves electromagnetic fields. In this approach, the capsule contains coils or conductive materials that interact with external electromagnetic fields to generate motion. For example, a rotating magnetic field can be used to rotate a magnet embedded in the capsule, which in turn drives the capsule forward. This method has been successfully demonstrated in laboratory settings, but like other external methods, it faces challenges related to the strength of the fields required and the complexity of the control systems involved [14].
Localization of capsule endoscopes is a critical aspect of their functionality, especially as the technology evolves from purely diagnostic devices to tools that can potentially perform therapeutic interventions. The ability to precisely track the position and orientation of the capsule as it travels through the gastrointestinal (GI) tract is essential for accurate diagnosis and, in the future, for ensuring that the capsule can be effectively guided to specific areas requiring closer examination or treatment. Without accurate localization, the information provided by the capsule’s imaging system may be less valuable, as clinicians cannot be sure of the exact location of abnormalities, such as ulcers, tumors, or areas of bleeding. Additionally, if the capsule is to perform tasks beyond imaging—such as delivering drugs or performing biopsies—knowing its exact position within the body becomes even more critical.
Currently, most commercial capsule endoscopy systems rely on rudimentary localization techniques, primarily based on triangulating the strength of radiofrequency (RF) signals received from the capsule by sensors placed on the patient’s body. These sensors, typically positioned around the abdomen, detect the signal strength emitted by the capsule, and based on this information, an approximate location of the capsule can be estimated. However, this method is often imprecise, providing only rough estimates of the capsule’s position, typically within a few centimeters. While this level of accuracy may be sufficient for general diagnostics, it is inadequate for more complex applications, such as targeting specific areas for biopsy or delivering localized treatments.
Magnetic localization systems have been proposed as a more accurate alternative to RF-based methods. In these systems, a small permanent magnet is embedded within the capsule, and its position is tracked by external sensors that detect changes in the magnetic field as the capsule moves through the body. The magnetic field generated by the magnet can be mapped, and with sophisticated algorithms, the position and orientation of the capsule can be determined in real time. This approach has the potential to significantly improve the accuracy of localization, with errors reduced to within a few millimeters, providing a much clearer picture of where the capsule is at any given time.
Magnetic localization also offers the advantage of being less affected by the body’s internal environment compared to RF signals, which can be distorted by tissues, fluids, and movement. However, there are still challenges associated with magnetic localization. For example, the strength of the magnetic field diminishes rapidly with distance, which means that sensors need to be placed relatively close to the body to maintain accuracy. Moreover, external magnetic fields—such as those from other medical equipment—can interfere with the system, complicating the localization process.
Another promising approach involves the use of imaging techniques like X-rays, computed tomography (CT) scans, and magnetic resonance imaging (MRI) to track the capsule’s position. These methods can provide highly accurate localization by directly visualizing the capsule as it moves through the GI tract. However, their practicality in real-time tracking is limited, as these imaging techniques are generally stationary and cannot continuously monitor the capsule throughout its journey. Moreover, exposure to ionizing radiation in X-ray or CT scans poses risks, making these methods unsuitable for routine use during the entire duration of the capsule’s transit through the GI tract.
Ultrasound-based localization is another technique under investigation, which could provide a non-invasive and radiation-free method for tracking the capsule. This method would involve external ultrasound sensors detecting the location of the capsule based on its reflections and movements within the body. However, ultrasound localization is still in the experimental stage and faces several challenges, including the need for real-time processing and handling the complex acoustics of the human body.
In addition to these hardware-based approaches, software improvements are also being pursued to enhance localization accuracy. Advanced algorithms, such as machine learning and artificial intelligence (AI), are being explored to process sensor data more effectively and predict the capsule’s location with higher precision. These algorithms can integrate data from multiple sources—such as magnetic, RF, and imaging data—to create a more comprehensive model of the capsule’s movement and position. By analyzing patterns in the capsule’s trajectory, these systems can compensate for some of the inaccuracies and limitations of individual localization methods, providing a more reliable and accurate estimate of the capsule’s location.
As capsule endoscopy technology advances toward becoming an active tool that can not only diagnose but also treat gastrointestinal diseases, precise localization will become even more critical. For example, in future applications where capsules might be equipped with tools for performing biopsies or administering localized treatments, doctors will need to know exactly where the capsule is located to avoid damaging surrounding tissues and to ensure that the correct area is targeted.
Localization will also play a crucial role in controlling the movement of active capsules. If the capsule can be externally guided, either through magnetic actuation or other means, real-time localization will provide the feedback necessary to adjust the capsule’s course, ensuring that it reaches the desired location within the GI tract. The integration of actuation and localization technologies will likely be one of the most significant challenges in the development of the next generation of capsule endoscopes, as both systems must work in tandem to provide precise control and positioning without interfering with each other.
Ultimately, the goal is to achieve highly accurate, real-time localization that is non-invasive, reliable, and unaffected by external or internal factors. As research continues, it is expected that the combination of advanced sensors, imaging technologies, and AI-based software will enable future capsule endoscopes to provide not only unprecedented diagnostic capabilities but also targeted therapeutic interventions.
Capsule endoscopy has revolutionized gastrointestinal diagnostics by offering a non-invasive, patient-friendly method for examining the entire digestive tract, particularly the small intestine. While current capsule endoscopes are limited to passive movement and diagnostic functions, ongoing research into actuation and localization technologies promises to expand their capabilities. The development of active capsules, capable of controlled navigation and real-time therapeutic interventions, represents the next frontier in this field. Overcoming challenges related to power supply, movement control, and precise localization will be crucial to realizing the full potential of capsule endoscopy, paving the way for more comprehensive and effective GI care.
Than, Trung Duc, et al. “A review of localization systems for robotic endoscopic capsules.” IEEE transactions on biomedical engineering 59.9 (2012): 2387-2399.
Liu, Lejie, Shahrzad Towfighian, and Amine Hila. “A review of locomotion systems for capsule endoscopy.” IEEE reviews in biomedical engineering 8 (2015): 138-151.
Yim, Sehyuk, and Metin Sitti. “3-D localization method for a magnetically actuated soft capsule endoscope and its applications.” IEEE Transactions on Robotics 29.5 (2013): 1139-1151.
Shamsudhin, Naveen, et al. “Magnetically guided capsule endoscopy.” Medical physics 44.8 (2017): e91-e111.
Chen, Dongmei, et al. “The force model of wireless active actuation for capsule endoscope in the GI tract.” 2007 IEEE International Conference on Robotics and Biomimetics (ROBIO). IEEE, 2007.
Lee, Cheong, et al. “Active locomotive intestinal capsule endoscope (ALICE) system: A prospective feasibility study.” IEEE/ASME Transactions on Mechatronics 20.5 (2014): 2067-2074.
Aghanouri, Mehrnaz, Ali Ghaffari, and Nasim Dadashi Serej. “Image based high-level control system design for steering and controlling of an active capsule endoscope.” Journal of Intelligent & Robotic Systems 94 (2019): 115-134.
Naser, Maged, Mohamed M. Naser, and Lamia H. Shehata. “Wireless Capsule Endoscopy (WCE).” International Journal of Progressive Sciences and Technologies 36.1 (2022): 150-167.
Than, Trung Duc, et al. “Enhanced localization of robotic capsule endoscopes using positron emission markers and rigid-body transformation.” IEEE Transactions on Systems, Man, and Cybernetics: Systems 49.6 (2017): 1270-1284.
Chen, Wenwen, et al. “Wireless powered capsule endoscopy for colon diagnosis and treatment.” Physiological measurement 34.11 (2013): 1545.
Sperry, Adam J., Jordan J. Christensen, and Jake J. Abbott. “Six-degree-of-freedom localization with a 3-axis accelerometer and a 2-axis magnetometer for magnetic capsule endoscopy.” IEEE Robotics and Automation Letters 7.2 (2022): 2110-2115.
Boroujeni, Pouria Sadeghi, et al. “Model-aided real-time localization and parameter identification of a magnetic endoscopic capsule using extended Kalman filter.” IEEE Sensors Journal 21.12 (2021): 13667-13675.
Abu-Kheil, Yasmeen, et al. “Vision and inertial-based image mapping for capsule endoscopy.” 2015 International Conference on Information and Communication Technology Research (ICTRC). IEEE, 2015.